This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
When proteins get stuck at the solid phase: Unlocking the secrets to brain diseases
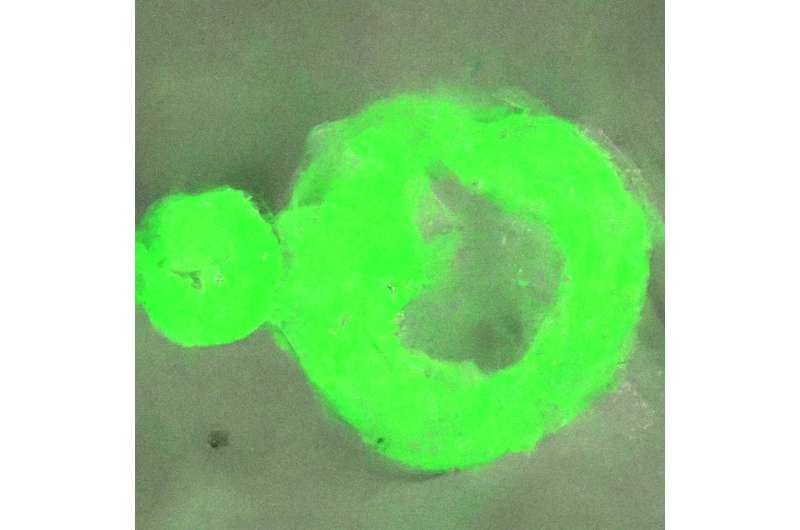
Many diseases affecting the brain and nervous system are linked to the formation of protein aggregates, or solid condensates, in cells from their liquid form condensate, but little is known about this process.
This liquid-to-solid transition can trigger the formation of what are called amyloid fibrils. These can further form plaques in neurons causing neurodegenerative diseases such as Alzheimer's.
Biomedical engineers at the University of Sydney, in collaboration with scientists at the University of Cambridge and Harvard University, have now developed sophisticated optical techniques to monitor at close range the process by which these protein aggregates form.
By testing a protein associated with amyotrophic lateral sclerosis—ALS disease, which affected astrophysicist Professor Stephen Hawking—the Sydney engineers closely monitored the transition of this protein from its liquid to solid phase.
"This is a huge step forward to understanding how neurogenerative diseases develop from a fundamental perspective," said Dr. Yi Shen, lead author of the research published in the Proceedings of the National Academy of Sciences (PNAS).
"We can now directly observe the transition of these critical proteins from liquid to solid at the nanoscale—a millionth of a meter in scale," said Dr. Daniele Vigolo, a senior lecturer in the School of Biomedical Engineering and a member of the University of Sydney Nano Institute.
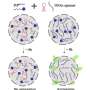
Proteins regularly form condensates during liquid-to-liquid phase separation in a wide range of critical and healthy biological functions, such as the formation of human embryos. This process assists biochemical reactions where protein concentrations are critical and also promotes healthy protein–protein interactions.
"However, this process also increases the risk of dysfunctional aggregation, where unhealthy aggregates of solid proteins form in human cells," said Dr. Shen, who is an ARC DECRA Fellow in the School of Chemical and Biomolecular Engineering and also a member of Sydney Nano.
"This can lead to aberrant structures associated with neurodegenerative diseases because the proteins no longer exhibit rapid reversibility back to liquid form. It is therefore crucial to monitor condensate dynamics, as they directly affect pathological states," she said.
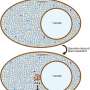
The world-first nanoscale optical observation of this process has allowed the team to determine that the transition from liquid to solid protein starts at the interface of the protein condensates. This window onto the phase transition also revealed that the internal structures of these protein agglomerates are heterogenous, where previously they were thought to be homogeneous.
Dr. Vigolo said, "Our findings promise to greatly improve our understanding of neurogenerative diseases from a fundamental perspective.
"This means a promising new area of research to better understand how Alzheimer's disease and ALS develops in the brain, affecting millions of people worldwide."
More information: Yi Shen et al, The liquid-to-solid transition of FUS is promoted by the condensate surface, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2301366120
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Sydney