This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Chinese scientists unravel cGNAT2 role in cyanobacteria
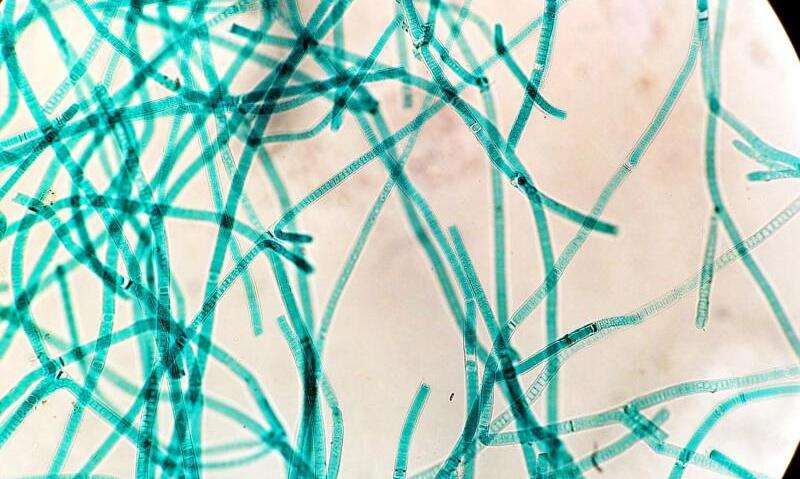
Cyanobacteria are a diverse group of gram-negative bacteria that conduct plant-like oxygenic photosynthesis and are thought to be the evolutionary ancestors of chloroplasts in higher plants. Lysine acetylation is an important regulatory posttranslational modification that controls photosynthesis and metabolic processes in cyanobacteria and plants.
Synechococcus PCC 7002 is a unicellular cyanobacterium that has been used as a model organism to study photosynthesis. A total of 802 acetylated proteins in Synechococcus PCC 7002 have been identified; however, the enzymes that govern reversible lysine acetylation in cyanobacteria remain largely unknown.
A research team led by Prof. Ge Feng from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences has illustrated the structure, function, and mechanism of lysine acetyltransferase in cyanobacteria, and identified acetylation in regulating the growth and photosynthesis of Synechococcus PCC 7002. The study was published in Plant Physiology.
In this study, the researchers identified 16 predicted lysine acetyltransferases (KATs) in the Synechococcus PCC 7002 genome using bioinformatic analyses, and tested these KATs using an acetylation-deficient E. coli strain devoid of all known acetylation mechanisms.
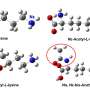
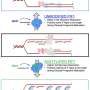
They discovered the presence of the lysine acetyltransferase cyanobacterial Gcn5-related N-acetyltransferase (cGNAT2) in cyanobacteria, and that the knockout of this gene significantly affected the photosynthesis in cyanobacteria.
Then, the researchers utilized AlphaFold2 to predict the structure of cGNAT2 and discovered that it forms a homodimeric structure. This protein has the capability to bind both substrate proteins and acetyl-CoA, enabling it to exert catalytic activity.
Through molecular docking, the binding region between cGNAT2 and acetyl-CoA was identified. Further confirmation was obtained through site-specific mutations and in vitro enzyme activity experiments, which demonstrated the critical role of eight specific amino acid residues in maintaining the enzyme's activity.
Based on acetyllysine enrichment and label-free quantitative (LFQ) acetylome techniques, the researchers demonstrated that cGNAT2 can catalyze lysine acetylation in NAD(P)H dehydrogenase J (NdhJ) to regulate its enzymatic activity in vivo and in vitro, which may underlie changes in cell growth and photosynthetic electron transport.
This study presents the first report of the lysine acetyltransferase cGNAT2 in cyanobacteria, elucidating its molecular mechanism in regulating substrate protein acetylation modifications in cyanobacteria. It will improve the understanding of the mechanisms underlying extensive lysine acetylation in cyanobacteria, as well as the mechanisms that regulate photosynthesis in photosynthetic organisms.
More information: Kun Jia et al, Deciphering the structure, function, and mechanism of lysine acetyltransferase cGNAT2 in cyanobacteria, Plant Physiology (2023). DOI: 10.1093/plphys/kiad509
Provided by Chinese Academy of Sciences