Researchers identify structure and function of first deacylase enzyme CddA in cyanobacteria
In a study published online in Plant Physiology, the research group led by Prof. Ge Feng from Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences identified the first deacylase enzyme CddA that has both deacetylase and depropionylase activities in cyanobacteria.
The researchers found that the loss of the gene cddA lead to slower growth and impaired linear and cyclic photosynthetic electron transfer.
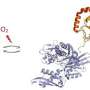
They determined its crystal structure at a resolution of 2.1 Å and established that it has a unique and characteristically folded α/β structure and a tube-like pocket likely playing a role in substrate binding.
By detecting an acyl binding site within CddA via site-directed mutagenesis, the researchers demonstrated that this site is essential for the deproprionylase activity of this enzyme.
Resorting to a proteomic approach, they next characterized in vivo protein acetylation and propionylation in Synechococcus 7002 and found that these modified proteins were highly enriched for photosynthetic and metabolic functionality. In addition, they demonstrated that CddA was capable of catalyzing in vivo and in vitro lysine depropionylation and deacetylation of fructose-1,6-bisphosphatase (F/SBPase), thereby regulating its enzymatic activity.
Multiple complex regulatory mechanisms govern the photosynthetic and metabolic pathways within cyanobacteria. While these mechanisms remain incompletely understood, there is increasing evidence indicating that a wide range of post-translational modification (PTMs) in both cyanobacteria and plants can regulate these processes, making the study of the enzymes regulating these processes of great interest.
Two enzyme families are responsible for regulating lysine acylation, lysine acyltransferases and deacylases. However, they are still not present in cyanobacteria. This study provides insight into the mechanisms globally regulating photosynthesis and carbon metabolism in cyanobacteria and potentially in other photosynthetic organisms as well.
More information: Xin Liu et al. Structural and functional insights into a lysine deacylase in the cyanobacterium Synechococcus sp. PCC 7002, Plant Physiology (2020). DOI: 10.1104/pp.20.00583
Provided by Chinese Academy of Sciences