This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Advancing viticulture: Pioneering transgene-free CRISPR genome editing in grapevines

Grapevine (Vitis vinifera L.) holds significant economic and cultural value, driving the need for rapid genetic improvement to meet climatic and market demands.
While traditional breeding is slow and genetically modified (GM) varieties face regulatory hurdles due to safety concerns, CRISPR/Cas9 has emerged as a promising tool for precise genome editing. Although successful in enhancing disease resistance and understanding gene functions in grapevines, CRISPR/Cas9 applications have so far resulted in GM plants, which is incompatible with current regulations governing GM plants in many countries.
Therefore, the current challenge lies in the refinement of CRISPR/Cas9 delivery methods to produce transgene-free, edited whole grapevine plants without unintended genetic changes.
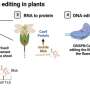
In October 2022, Horticulture Research published a perspective entitled "DNA-free genome editing in grapevine using CRISPR/Cas9 ribonucleoprotein complexes followed by protoplast regeneration."
In this study, a grapevine cell line of cv. Thompson Seedless was genetically engineered to overexpress GFP for genome editing. Through tissue culture techniques, ~10% of somatic embryos induced embryogenic callus expressing GFP after ∼10 weeks. These were used to extract protoplasts, achieving a protoplast-to-whole-plant regeneration efficiency of ~30%, and all of the isolated protoplasts were transgenic with GFP signal.
CRISPR/Cas9 ribonucleoproteins (RNPs) named RNP1, RNP2, RNP3 and RNP4 were then employed to knock out the GFP gene in these transgenic protoplasts. Among them, two embryos transfected with RNP2 and RNP4 were lacking GFP fluorescence, with an efficiency of 23% and 34% respectively from mature cotyledonary embryos to whole plants.
Post-CRISPR editing, two GFP-negative embryos were identified, suggesting successful gene knockout. Further analysis confirmed targeted GFP gene mutations, with a frameshift causing the loss of GFP expression in these plants.
In conclusion, this study effectively demonstrates the integration of somatic embryogenesis and CRISPR/Cas9 genome editing in grapevines, paving the way for more widely accepted modifications in agriculture.
More information: Samaneh Najafi et al, DNA-free genome editing in grapevine using CRISPR/Cas9 ribonucleoprotein complexes followed by protoplast regeneration, Horticulture Research (2022). DOI: 10.1093/hr/uhac240
Journal information: Horticulture Research
Provided by NanJing Agricultural University