This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists unlock the secrets of nitrogen's solid phase
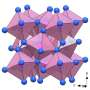
![Crystal structure of ζ-N2 at 63 GPa and that of ε-N2 at the same density. The structures from (a) to (d) are of ζ-N2 at 63 GPa and those in (e) and (f) of ε-N2. a Structural motif of ζ-N2 presented by chains of apexes-sharing triangular bipyramids (in red) aligned along the [25 0 −12] direction (a unit cell is outlined); the corners of the pyramids appear at the centers of mass of N2 molecules. The blue, pink, orange and green spheres correspond to the N1, N2, N3, and N4 atoms; (b) the structure of ζ-N2 viewed along the [25 0 −12] direction; (c, d) the structure of ζ-N2 in two orientations ([0 0 1] and [−251 −195 21], respectively) helpful for visualizing its details. e The structure of ε-N2 viewed along the c direction (the blue and red spheres represent the N1 and N2 atoms, respectively); (f) the structure of ε-N2 in the orientation simplifying a comparison with the structure of ζ-N2 shown in (c, d). Blue and red circles help underline the differences between ε-N2 and ζ-N2. The crystallographic data for ζ-N2 at 63 GPa has been submitted under the deposition number CCDC 2237807. Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-41968-2 Scientists unlock the secrets of nitrogen's solid phase](https://scx1.b-cdn.net/csz/news/800a/2023/scientists-unlock-the.jpg)
In a groundbreaking study now published in the journal Nature Communications, the mysteries of nitrogen's solid phases have been solved, shedding light on its complex behavior.
The research, led by the University of Bayreuth, in collaboration with scientists from the University of Edinburgh, U.K., and the University of Linköping, Sweden, provides unprecedented insights into the gradual molecular-to-polymeric transformation of nitrogen and the formation of amorphous nitrogen. This paves the way for advances in materials science and high-pressure physics.
At ambient pressure and temperature, nitrogen is gas and is found in the form of an N2 molecule (N≡N) composed of an extremely strong triple-bond. When extreme pressures are applied to molecular gaseous nitrogen, it first becomes liquid and then a solid at about 2.5 GPa (i.e., 25,000 times the atmospheric pressure).
For more than a century, scientists have delved into solid phases of molecular nitrogen, as knowledge of the chemico-physical mechanisms underpinning transformations in nitrogen is vital in testing and refining theories of solid-state sciences.
The Zeta-N2 phase of nitrogen, existing between 60 and 115 GPa, is a critical piece of the puzzle for understanding nitrogen's molecular to polymeric transition. However, despite a large number of investigations, its crystal structure (i.e., the nitrogen molecules' arrangement) was hitherto unknown—and key to deciphering nitrogen's odd behavior.
The research team, led by Dominique Laniel (University of Edinburgh) and Natalia Dubrovinskaia and Leonid Dubrovinsky (the University of Bayreuth) employed an experimental technique newly developed in Bayreuth to successfully determine the crystal structure of Zeta-N2.
The researchers squeezed molecular nitrogen in diamond anvil cells to extreme pressures between 60 and 85 GPa, such as those prevailing in the Earth's mantle. By applying laser heating up to 2,000°C, they were able to recrystallize high-quality submicrometer-size grains of Zeta-N2. Their crystal structure was solved and refined from synchrotron single-crystal X-ray diffraction. With these experimental findings in hand, theoreticians at the University of Linköping (Sweden) gained further insights into nitrogen's unique polymerization process.
The implications of this research extend beyond nitrogen itself, offering a deeper understanding of molecular transformations under extreme conditions. The findings pave the way for advancements in solid-state sciences, materials science and high-pressure physics. The researchers have improved the methods of studying the properties of functional materials used in electronics, computer chips, semiconductors, solar cells, batteries, lighting, metals or insulators.
More information: Dominique Laniel et al, Structure determination of ζ-N2 from single-crystal X-ray diffraction and theoretical suggestion for the formation of amorphous nitrogen, Nature Communications (2023). DOI: 10.1038/s41467-023-41968-2
Journal information: Nature Communications
Provided by Bayreuth University