This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers model cell behavior called 'coiling' to understand cancer dynamics
In any fight, knowing your enemy is critical to staging a defense. The fight to stop cancer or to accelerate wound healing is no exception. The research teams at Virginia Tech and the Weizmann Institute of Israel, along with partners worldwide, are pursuing a deeper understanding of how cells move and spread throughout a living body.
Professor Amrinder Nain at Virginia Tech builds nanoscale suspended bridges to study cell migration. Professor Nir Gov at the Weizmann Institute develops the theoretical and computational framework for how cells migrate on curved surfaces. Their collaborative study combining state-of-the-art experiments and theory to examine cell "coiling" on fibers has been published in Nature Communications.
This study follows previous research partnering Gov and Nain for exploration of the inner mechanics of cancer. In that work, Nain and his partners from Virginia Tech, Japan, and Israel studied how a cell's biology affects the motion of brain cancer cells.
That work produced several novel discoveries, but chemistry and biology alone did not provide a complete picture. Needing a more holistic view of cellular behavior to understand how to halt cancer in its tracks, the team shifted from studying the inside of the cell to its outside, observing how it interacted with its environment.
Expanding the team for a new study
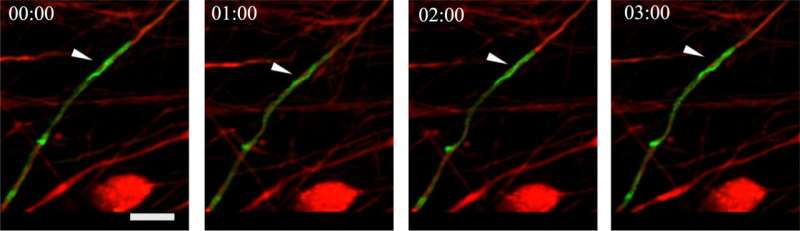
Nain and Virginia Tech colleague Bahareh Behkam had previously identified a cell behavior called coiling, in which a cell wraps itself around a fiber axis to migrate. They found that coiling was more pronounced in cancerous invasive cells than their non-tumorigenic counterparts. Knowing this, they set out to understand the underlying energetic principles governing that coiling behavior.
Again needing Gov's expertise, the team launched a new collaborative study with the team from Israel, aimed at discovering how a cell moves using its protrusions, or arm-like structures that extend outward from the front of a cell's soft body.
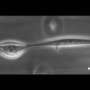
Nain and his collaborators knew these arms not only allow the cell to move, but also to grasp its environment and pull itself forward. The trick was to observe them in 3D at sufficient resolution. Virginia Tech team member Christian Hernandez-Padilla devised fiber networks and imaging strategies to capture coiling events. Nain then contacted Hari Shroff and Harshad Vishwasrao at the National Institutes of Health (NIH) to ask about using their lattice-light sheet advanced microscope.

"We challenged ourselves to determine if coiling could be clearly observed in 3D for detailed measurements," said Nain. "All it took was a cold email to Hari at NIH, to which he was extremely receptive. We were jubilant as Christian's imaging data trickled in, showing cells coil on fibers in 3D."
In addition to the NIH, the team also reached out to Professor Konstantinos Konstantopoulos at Johns Hopkins University to generate specific cell lines used in the study; Professor Aleš Iglič at the University of Ljubljana, Slovenia, for computational modeling; and Professor Elior Peles at the Weizmann Institute of Science for demonstrating coiling in vivo.
Understanding cell migration requires knowing how cells curve around fibrous ropes—the suspended bridges on which they eventually move. Nain's expertise includes building nanoscale cellular suspended roadways that are fibrous. Compared to the flat landscape of a Petri dish, these fibers are much closer to the landscape of living tissues. By partnering with other experts, the team set the stage for illustrating how cells move inside a body, which could lead to new strategies to stop cancer cells or accelerate wound healing.
A twisted grip: Work from Blacksburg
To propel itself, a cell's jelly-like body first produces the tentacle-like protrusions. These cellular arms can grab onto things by twisting around fibers in the tissues surrounding them. But this behavior has rarely been studied before.
"Recent imaging studies inside the body have shown cancer cells moving along individual fibers and navigating through varying fibrous architectures by reaching out and grabbing the fibers," Nain said. "We combined our experiments with Nir's computational models to understand the energetics of coiling. This had never been attempted before, and it challenged our groups."
The group studied coiling on suspended fibers of various diameters, including flat ribbons pioneered in the Behkam lab. Researchers found that as a cell settled onto a fiber, its tentacle wrapped a few times around the fiber, giving the cell a firm grip. Hernandez-Padilla performed imaging at the NIH and developed the framework to quantify 3D coiling events from the voluminous data recorded.
The coiling: Work from Israel
In Israel, postdoctoral fellow Rajkumar Sadhu created a theoretical model that describes how a cell may get its shape and move when outside forces act on its membrane. Gov's team found that energy minimization was a major driver. Picture a membrane trying to remain as flat as possible, avoiding sharp corners that would require more energy to navigate.
Complicated shapes such as the coiling result from protein complexes, themselves curved, bending the membrane as it follows their shape. Curved proteins also connect with the cytoskeleton, the structural component giving the cell its shape. The cytoskeleton grows and pushes outward during cellular movement, driving the protrusions.
These forces, arising from energy conservation and cytoskeleton dynamics, are responsible for the coiling. The model correctly predicted that the coiling would cease when the fiber had sharp corners, as in the case of the flat ribbons.
Collaborative work is key in biology
While this balance of energy between movement and cell biology happens in very small ways, it holds enormous implications for the future. Understanding how cells behave in their environment opens the door to understanding cell migration during developmental, disease, and repair biology.
In addition to the scientific advances of this project, Gov commented on the value of this work to the collaborative enterprise.
"This collaboration already produced several publications and demonstrates how science is being done today through collaborations between people from different countries, continents, and ethnic and national backgrounds," he said. "Beyond the curiosity and love of science, what unites us are the liberal ideals of freedom, human rights, and mutual respect and solidarity between all people."
More information: Raj Kumar Sadhu et al, Experimental and theoretical model for the origin of coiling of cellular protrusions around fibers, Nature Communications (2023). DOI: 10.1038/s41467-023-41273-y
Journal information: Nature Communications
Provided by Virginia Tech