This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study finds ways to enhance transcription factor activity
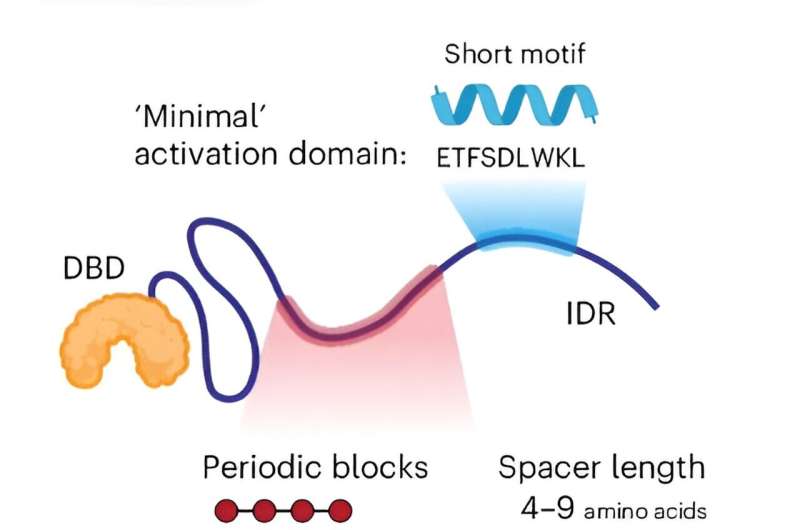
Transcription factors regulate gene expression by binding specific sequences on DNA, which is an essential step to producing messenger RNAs from protein-coding genes. Denes Hnisz's lab, in collaboration with Martin Vingron's lab at the MPIMG, has discovered that human transcription factors don't typically use their full potential. Instead, important protein regions within transcription factors encode chemical features that generate submaximal transcriptional activity.
The findings, published in Nature Cell Biology, suggest simple ways to engineer natural transcription factor variants with elevated or "optimized" activity, with potential applications for regenerative therapy.
Every cell contains the same set of genetic information, but not all of the genes are expressed in every cell. The specific patterns of gene expression in cells make a neuron look different and perform different functions than cells in other organs or tissues.
Transcription factors guide the formation of tissues and organs during development, and help maintain the identity of adult cells, by binding specific DNA sequences and activating or repressing them. They face the complex problem of balancing which genes to bind and how much to activate them. How transcription factors perform and balance these functions has been a long-standing mystery.
Insights from the past few years suggested that transcription factors may exert some of their functions through forming liquid-like proteinaceous droplets, called condensates.
"We and others have shown that inhibiting the ability of transcription factors to form condensates also reduces their activity in cells," explains group leader Hnisz.
"In our study, we now did the opposite: We enhanced the ability of transcription factors to form liquid-like droplets and found that this, in turn, improved their activity." However, this improvement comes with a trade-off.
Improving patterns
In 2020, scientists made an observation that would provide inspiration for the current study.
"It was shown that in RNA-binding proteins, periodically spaced amino acids contribute to the ability of the proteins to form liquid-like condensates. We asked whether such periodic patterns also exist in transcription factors," says Alexandre Magalhães, a scientist in the Hnisz lab and one of the study's first authors.
In collaboration with Martin Vingron's lab at the MPIMG, the researchers developed bioinformatics approaches to identify these periodically arranged chemical features, so-called aromatic residues, in about 1,500 human transcription factors. Starting with the protein sequences of the transcription factors, the team looked for the positions of the aromatic amino acids and quantified how regularly they were arranged.
"We found some traces of periodicity, but for the vast majority of factors, the patterning was quite imperfect, leaving room for improvement. We started moving the amino acids around computationally to make the spacing of the aromatic residues more uniform," explains Hnisz. Moving from computers to cells, the scientists then tested the effects of the improved protein sequences.
"The transcription factors became more active. To our surprise, however, they also became less specific in binding DNA," says Hnisz.
An evolutionary trade off
"Our model is that the functional features of transcription factors, such as DNA binding specificity or activation strength are not maximal, because they are optimized for the overall contribution of the transcription factor to evolutionary fitness," explains Julian Naderi, a Ph.D. student and another of the paper's first authors.
"We can now show that the reason for this is that their features are in a trade-off, meaning that if you improve one, the other gets weaker and vice versa."
However, this provides an opportunity to adjust the balance between the two properties. "If you know the trade-offs, it is conceivable to tweak transcription factors, depending on which function is needed more in an application," he adds.
One possible application could be in regenerative medicine, where scientists are trying to replace damaged or lost tissue with a patient's own cells. Since only a few transcription factors can maintain a particular cell type, it is a tempting approach to reprogram other cells into the desired type by upregulating these factors.
Such approaches are currently in pre-clinical testing for example, to repair brain damage after stroke by reprogramming astrocytes into neurons.
"We have shown in the study that with a minor sequence adjustment in a single transcription factor, we can significantly enhance its ability to convert cells into neurons in a cell culture dish," says Hnisz.
"It will be very exciting to test whether the approach works in a stroke model."
More information: Julian Naderi et al, An activity-specificity trade-off encoded in human transcription factors, Nature Cell Biology (2024). DOI: 10.1038/s41556-024-01411-0
Journal information: Nature Cell Biology
Provided by Max Planck Society