Nanoscale forces measured in aortic smooth muscle cells tell story of disease
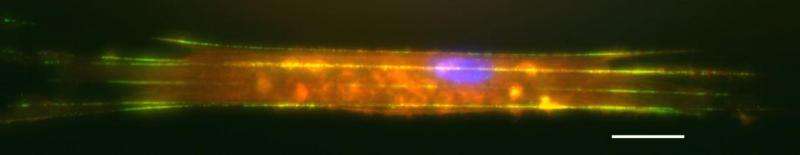
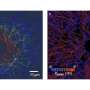
Researchers from Virginia Tech and the University of Pittsburgh have collaborated to employ a novel nanoscale fibrous system that can measure the tiny forces exerted by and upon individual cells with extreme precision. The team hopes that this platform, which investigators call nanonet force microscopy (NFM), will provide new knowledge about smooth muscle cell biology that could have implications for treating cardiovascular disease, which is still a leading cause of death in the United States.
The results of investigations on cells using this platform appear in the "Forces" issue of the journal Molecular Biology of the Cell, in the article "Nanonet Force Microscopy for Measuring Forces in Single Smooth Muscle Cells of Human Aorta," published July 7, 2017.
The main goal of this current study, said Julie Phillippi, assistant professor at the University of Pittsburgh Department of Cardiothoracic Surgery whose laboratory provided healthy human patient smooth muscle cells for the study, was to quantify forces that healthy cells experience in various conditions of stress. The fibrous nanonet itself was designed in the mechanical engineering laboratory of Amrinder Nain, associate professor at Virginia Tech and member of the American Society for Cell Biology. Forces measured using NFM, Nain said, include forces exerted by the cells themselves and forces exerted by the environment on the cells. "Everything in nature exerts and experiences a physical force," said Nain. "This platform measures both simultaneously."
Phillippi said that previous work tested the mechanical strength of whole aortic tissue and understanding the single cell biomechanics is vitally important. Single-cell studies provide insight into the proteins involved in the fleeting so-called focal adhesions that most cells make as they move around their microenvironment. The NFM assembly aims to mimic, in as physiologically relevant a way as possible, what cells endure within the collagen fibers of the extracellular matrix (ECM)—the matrix that supports cell growth in living things. Tweaking the artificial matrix by changing fiber diameter, density, and spacing in a controlled and repeatable manner, as well as using cells from diseased patients at different disease severities, will allow Phillippi and Nain to simulate the conditions experienced by cells in many realistic situations.
"We have looked very closely at how the collagen and elastin fibers in the ECM are arranged and the micro-architecture and everything points to these microstructural defects in the ECM contributing to the weakening of the aortic walls and the ballooning of the vessel," said Phillippi. "What we don't know is, are these ECM proteins arranged that way from birth or is it something that happens over time? Or is it both? What role do the cells play? This engineered platform will allow us to answer some of those questions." Furthermore, Nain said, NFM could reveal the heterogeneity of cells taken from the same patient or from different patients with the same disease state down to the single-cell resolution.
Next steps for Phillippi and Nain include testing cells from the Pittsburgh team's large repository of aortic specimens from patients, collected in collaboration with Thomas Gleason, Chief of the Division of Cardiac Surgery, University of Pittsburgh, to establish a database of baseline forces for many types of cells that researchers and clinicians can use to diagnose and treat disease. "The platform gives us the ability to create in vitro disease models with multiple layers of sophistication," said Phillippi.
In a broader context, the ability to achieve precise control on fiber diameter, spacing, and orientation to mimic native fibrous environments, will allow NFM to interrogate the push and pulls in a cell's journey in developmental, disease, and repair biology.
More information: Alexander Hall et al. Nanonet force microscopy for measuring forces in single smooth muscle cells of the human aorta, Molecular Biology of the Cell (2017). DOI: 10.1091/mbc.E17-01-0053
Journal information: Molecular Biology of the Cell
Provided by American Society for Cell Biology