This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Singling out a bacterium from the crowd
Bacteria are nearly ubiquitous and have tremendous impacts on human and ecological health. And yet, they remain largely mysterious to us. Princeton MOL faculty Zemer Gitai, Britt Adamson and Ned Wingreen launched a joint effort to develop new tools to help us better understand bacteria. Their work is described in a paper that appeared in the journal Nature Microbiology.
Bacteria are incredibly numerous and astoundingly diverse. The human gut contains as many bacterial cells as there are human cells in the entire body, spread across an estimated 500–1000 bacterial species. Bacteria also colonize surfaces and organisms all around us.
Individual bacteria of the same species may appear superficially similar but still exhibit diverse responses to stress, depending on the expression pattern of their genes—that is, which genes are actively being used to make end products such as proteins that affect cell behavior.
Historically, this type of variability among cells was hard to capture because the tools used to study bacteria were only suited to making generalizations about population-wide trends in gene expression. This obscured differences in gene expression that could explain important behaviors such as development of antibiotic resistance.
Recently, scientists have begun leveraging next-generation sequencing and associated techniques, such as single-cell RNA sequencing, that researchers can use to study gene expression in hundreds of thousands of individual cells simultaneously. However, many challenges remain.
For example, some of these new approaches are limited to studying only a narrow set of genes that we already know about, while others are hampered by technical issues that impair their sensitivity. The Princeton team, spearheaded by graduate student Bruce Wang, set out to overcome these limitations.
In order to observe which genes are being expressed in a cell, scientists need to look for a type of molecule called messenger RNA (mRNA for short), which appears when a protein-coding gene is being expressed. The problem arises because up to 97% of a bacterium's RNA molecules are a different type of RNA, called ribosomal RNA (rRNA).
Most approaches work by first tagging a random selection of all RNA molecules from the cell and then using deep sequencing to discover the tagged molecules' identities. The more abundant rRNA gets sequenced more often than mRNA, resulting in poor signal-to-noise ratios. Wang and his colleagues developed a way to remove ribosomal RNA sequences after the tagging step but before the sequencing step, which both enriches mRNA in the sample and reduces the expense of sequencing.
Wang also adopted a barcoding-based method to allow this approach to be applied to hundreds of thousands of cells at once.
Using this method, the Princeton group set out to investigate fundamental questions about bacterial biology. For example, they examined how E. coli bacteria respond to eight different types of antibiotics.
As expected, different types of antibiotics evoked different responses, depending on the mode of action of the antibiotic. However, the responses of individual cells were remarkably varied, with different subpopulations expressing different subsets of genes. Further study of the genes involved could help scientists develop more effective antibiotics or antibiotic combinations targeting these genes.
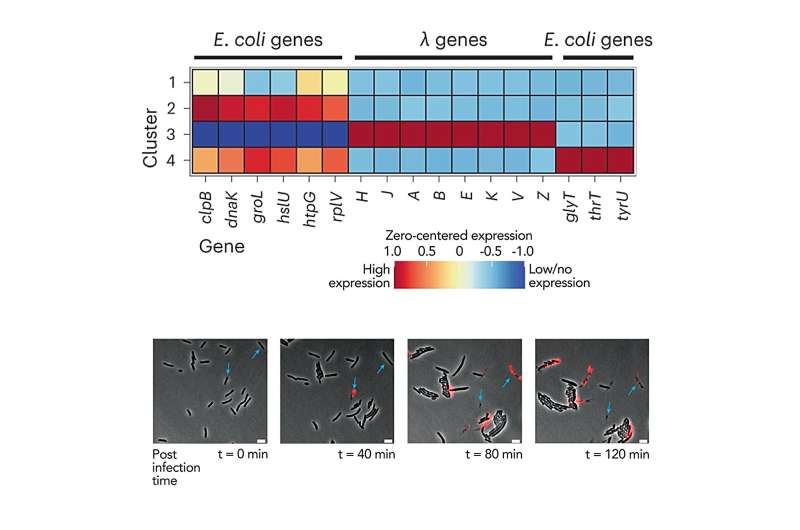
The team also probed how bacteria respond to infections by a virus called bacteriophage λ. Surprisingly, they found that only a third of the bacteria actually become infected by λ and begin producing its proteins, even when the phage outnumbers bacteria by 100:1. This highlights how methods that only look at responses of a population as a whole can obscure potentially important biological complexities.
This exciting new technology, which the authors call M3-seq (short for "massively parallel, multiplexed, microbial sequencing"), is not limited to use on single species of bacteria. It can also be used to study multispecies communities of bacteria like those found in the human microbiome, opening the door to new realms of inquiry that the team is eager to explore.
More information: Bruce Wang et al, Single-cell massively-parallel multiplexed microbial sequencing (M3-seq) identifies rare bacterial populations and profiles phage infection, Nature Microbiology (2023). DOI: 10.1038/s41564-023-01462-3
Journal information: Nature Microbiology
Provided by Princeton University