This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists make methanol at room temperature
A more sustainable method of creating methanol—a key component of fuels, plastics, and medicines—has been developed by Cardiff University scientists and an international team of collaborators.
The process, which uses a highly active catalyst, converts oxygen and the natural gas methane into methanol at room temperature without the need for external energy sources such as light or electricity.
The breakthrough builds on the Cardiff team's efforts to move away from expensive and energy-intensive processes by developing new catalytic methods with industry and promoting the use of catalysis as a sustainable 21st century technology.
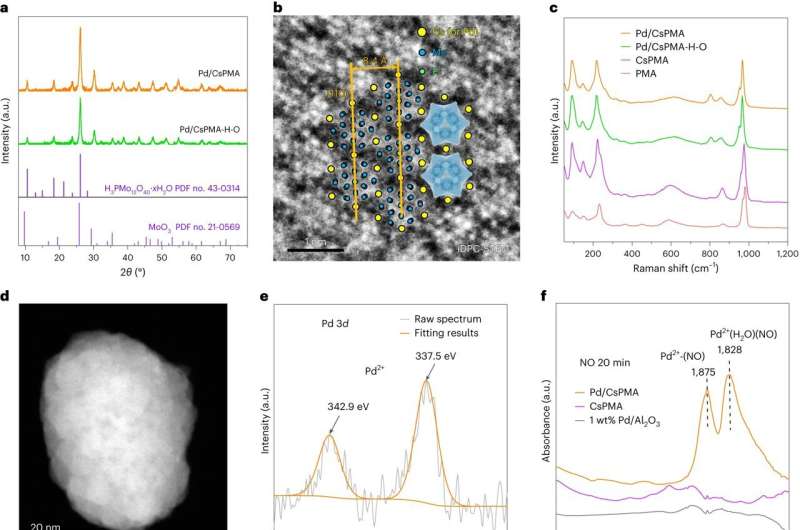
Their findings, published in an article titled "H2-reduced phosphomolybdate promotes room-temperature aerobic oxidation of methane to methanol" in Nature Catalysis, represent a significant step toward cleaner, greener methanol production which, with further development, could be used in industrial processes worldwide.
Professor Graham Hutchings, Regius Professor of Chemistry at Cardiff University and a collaborating author on the paper said, "Identifying new and effective catalysts for methanol synthesis from methane is of crucial importance to provide new pathways for the modern chemical industry."
The study is an international collaboration between the Max Planck Center on the Fundamentals of Heterogeneous Catalysis, the newly established Net Zero Innovation Institute at Cardiff University and institutions overseas.
Working out of state-of-the-art labs at Cardiff University's Translational Research Hub, the Cardiff team shared their expertise in catalyst design and advanced characterization techniques, which played a fundamental role in understanding how the catalyst operates and how its lifetime can be extended.
Co-author Dr. Andrea Folli, University Research Fellow in Electrocatalysis at the Net Zero Innovation Institute, added, "The discovery may be a significant step towards a sustainable methanol-based fuel economy, using abundant methane as the feedstock.
"Simultaneously, it provides the opportunity to establish a circular economy involving a critical greenhouse gas.
"The global warming potential of methane is 25 times the one of carbon dioxide so this is a crucial step in achieving net zero by 2050."
More information: Sikai Wang et al, H2-reduced phosphomolybdate promotes room-temperature aerobic oxidation of methane to methanol, Nature Catalysis (2023). DOI: 10.1038/s41929-023-01011-5
Journal information: Nature Catalysis
Provided by Cardiff University