This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers describe advances in mass spectrometry analysis to improve identification of glycopeptides
Glycosylation is the attachment of carbohydrates to the backbone of a protein through an enzymatic reaction. It plays a critical role in determining protein structure, function and stability. A protein that is glycosylated is known as a glycoprotein. The two most common types of protein glycosylation are known as N-glycosylation and O-glycosylation.
A new study by researchers at Boston University Chobanian & Avedisian School of Medicine set out to improve on existing methods to identify glycopeptides and found that conventional mass spectrometry methods were sufficient to identify peptides with one glycan (essential biomolecules serving structure, energy storage and system regulatory purposes) but that an additional step is necessary for identifying peptides with two or more glycans.
Because one protein can carry many glycans, it can be difficult or impossible to generate peptides that only carry one glycan. The ability to identify peptides with multiple glycans is key to defining different glycoforms of the protein to understand its biological function and the role in the disease.
Due to the lack of information about glycoproteins and how they change during development and disease, corresponding author Manveen K. Sethi, Ph.D., research assistant professor of biochemistry & cell biology, believes they may be missing promising therapeutic avenues. "A more thorough and specific understanding of how glycosylation affects disease, specifically site-specific alterations, may provide novel targets in diseases that currently lack effective treatment options," she says.
"Advances in technology to confidently identify densely glycosylated peptides would assist in differentiating healthy and diseased states on the basis of site-specific glycosylation and thus, enabling the use of glycoproteins as marker and therapeutic target in different diseases," Sethi explains.
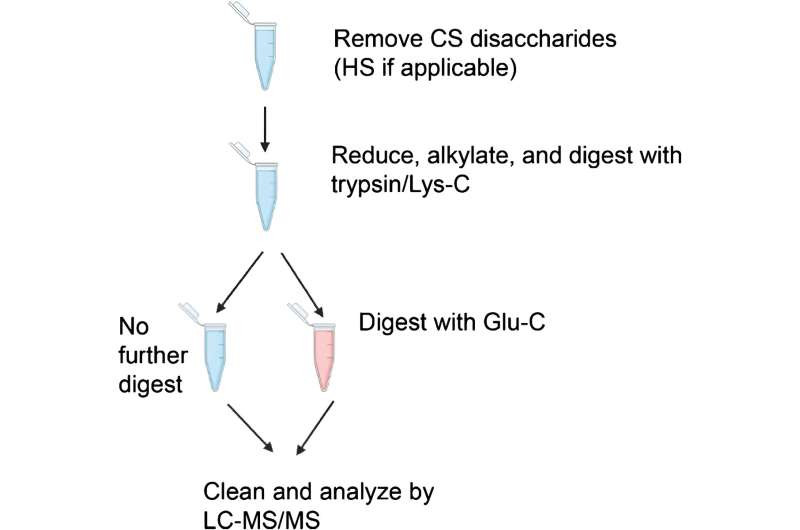
Using four different standard proteins, the researchers followed different enzymatic digestion protocols prior to mass spectrometry analysis. For mass spectrometry analysis they used a conventional higher-energy collisional dissociation (HCD) method and compared it to a more recently developed method—electron transfer/higher energy collisional dissociation (EThcD). They then compared the number of glycopeptides identified in the different digestion protocols and in the mass spectrometry methods.
While they found that that HCD is sufficient for glycopeptide identification, EThcD is often necessary to find glycans, particularly in the case of multiply-glycosylated peptides.
These findings appear online in the journal Analytical and Bioanalytical Chemistry.
More information: Margaret Downs et al, Analysis of complex proteoglycans using serial proteolysis and EThcD provides deep N- and O-glycoproteomic coverage, Analytical and Bioanalytical Chemistry (2023). DOI: 10.1007/s00216-023-04934-x
Journal information: Analytical and Bioanalytical Chemistry
Provided by Boston University School of Medicine