Reversible chemoenzymatic labeling strategy enables in-depth analysis of protein O-GlcNAcylation
O-linked β-N-acetylglucosamine (O-GlcNAcylation), an important post-translational modification (PTM) of proteins, is involved in various biological functions.
The reversible modification of O-GlcNAc confers on-off protein functions during biological processes. Aberrations of O-GlcNAcylation are closely associated with many metabolic diseases along with the invasion and metastasis of several tumors.
Recently, a research team led by Prof. Ye Mingliang and Prof. Qin Hongqiang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. Huang Wei from the Shanghai Institute of Materia Medica of CAS, developed a new strategy of reversible chemoenzymatic labeling of O-GlcNAc glycopeptides, which enabled in-depth analysis of protein O-GlcNAcylation.
Their findings were published in Angewandte Chemie on Mar. 14.
To enable the proteome-wide analysis of O-GlcNAcylation, it is essential to selectively enrich glycopeptides from the digests of complex samples.
Many researchers have sought the enrichment of O-GlcNAcylated peptides before analysis by liquid chromatography with tandem mass spectrometry (LC-MS/MS). However, most of the approaches suffer from weak binding affinity or bulky tags, which interfere with the enrichment and identification of O-GlcNAcylated peptides.
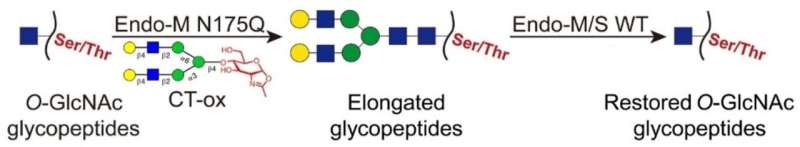
In this newly developed strategy, the O-GlcNAc moieties were ligated with long N-glycans using an Endo-M mutant, which enabled the enrichment of the labeled glycopeptides by hydrophilic interaction liquid chromatography (HILIC). Then, the attached glycans on the enriched glycopeptides were removed by wild-type Endo-M/S to restore the O-GlcNAc moiety.
Compared with the classic chemoenzymatic labeling, this approach enabled the tag-free identification and eliminated the interference of bulky tags in glycopeptide detection.
Moreover, by using this method, the researchers identified 657 potential O-GlcNAc glycosites from only 0.4 mg of HeLa cell nuclear proteins, which was needed only 1/10 of protein samples for a comparable O-GlcNAcylation analysis, indicating the high sensitivity of this method.
In total, they identified 1,414 glycosites from just 1.1 mg of protein samples, and 45% of them were not included in O-GlcNAcAltas of all human samples in the past 35 years, which enhanced the analysis coverage of protein O-GlcNAcylation.
"This tag-free enrichment strategy represents a unique avenue for proteome-wide analysis of O-GlcNAcylation and promotes the mechanism studies," said Prof. Ye.
More information: Yao Chen et al, Endo‐M Mediated Chemoenzymatic Approach Enables Reversible Glycopeptide Labeling for O ‐GlcNAcylation Analysis, Angewandte Chemie (2022). DOI: 10.1002/ange.202117849
Journal information: Angewandte Chemie
Provided by Chinese Academy of Sciences