This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Nanopore technology achieves breakthrough in protein variant detection
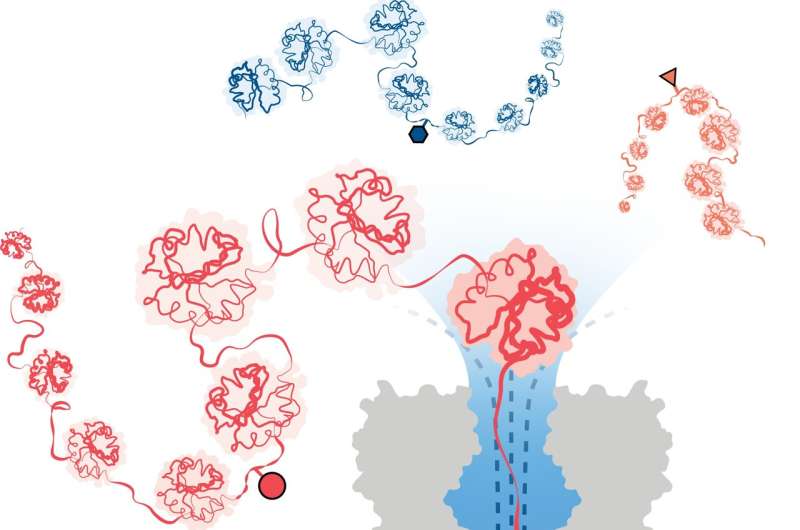
A team of scientists led by the University of Oxford have achieved a significant breakthrough in detecting modifications on protein structures. The method, published in Nature Nanotechnology, employs innovative nanopore technology to identify structural variations at the single-molecule level, even deep within long protein chains.
Human cells contain approximately 20,000 protein-encoding genes. However, the actual number of proteins observed in cells is far greater, with over 1,000,000 different structures known. These variants are generated through a process known as post-translational modification (PTM), which occurs after a protein has been transcribed from DNA.
PTM introduces structural changes such as the addition of chemical groups or carbohydrate chains to the individual amino acids that make up proteins. This results in hundreds of possible variations for the same protein chain.
These variants play pivotal roles in biology, by enabling precise regulation of complex biological processes within individual cells. Mapping this variation would uncover a wealth of valuable information that could revolutionize our understanding of cellular functions. But to date, the ability to produce comprehensive protein inventories has remained an elusive goal.
To overcome this, a team led by researchers at the University of Oxford's Department of Chemistry has successfully developed a method for protein analysis based on nanopore DNA/RNA sequencing technology. In this approach, a directional flow of water captures and unfolds 3D proteins into linear chains that are fed through tiny pores, just wide enough for a single amino acid molecule to pass through.
Structural variations are identified by measuring changes in an electrical current applied across the nanopore. Different molecules cause different disruptions in the current, giving them a unique signature.
The team successfully demonstrated the method's effectiveness in detecting three different PTM modifications (phosphorylation, glutathionylation, and glycosylation) at the single-molecule level for protein chains over 1,200 residues long. These included modifications deep within the protein's sequence. Importantly, the method does not require the use of labels, enzymes or additional reagents.
According to the research team, the new protein characterization method could be readily integrated into existing portable nanopore sequencing devices to enable researchers to rapidly build protein inventories of single cells and tissues. This could facilitate point-of-care diagnostics, enabling the personalized detection of specific protein variants associated with diseases including cancer and neurodegenerative disorders.
Professor Yujia Qing (Department of Chemistry, University of Oxford), contributing author for the study, said, "This simple yet powerful method opens up numerous possibilities. Initially, it allows for the examination of individual proteins, such as those involved in specific diseases. In the longer term, the method holds the potential to create extended inventories of protein variants within cells, unlocking deeper insights into cellular processes and disease mechanisms."
Professor Hagan Bayley (Department of Chemistry, University of Oxford), contributing author and co-founder of Oxford Nanopore Technologies, added, "The ability to pinpoint and identify post-translational modifications and other protein variations at the single-molecule level holds immense promise for advancing our understanding of cellular functions and molecular interactions. It may also open new avenues for personalized medicine, diagnostics, and therapeutic interventions."
This work was carried out in collaboration with the research group of mechanobiologist Sergi Garcia-Maynes at King's College London and the Francis Crick Institute.
More information: Pablo Martin-Baniandres et al, Enzyme-less nanopore detection of post-translational modifications within long polypeptides, Nature Nanotechnology (2023). DOI: 10.1038/s41565-023-01462-8
Journal information: Nature Nanotechnology
Provided by University of Oxford