This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Accelerating chemical reduction of carbon dioxide with ultrathin layers of tin disulfide
Researchers at Kanazawa University report in ACS Nano how ultrathin layers of tin disulfide can be used to accelerate the chemical reduction of carbon dioxide—a finding that is highly relevant for our quest towards a carbon-neutral society.
Recycling carbon dioxide (CO2) released by industrial processes is a must in humanity's urgent quest for a sustainable, carbon-neutral society. For this purpose, electrocatalysts that can efficiently convert CO2 into other, less impactful chemical products are widely researched today. A category of materials known as two-dimensional (2D) metal dichalcogenides are candidate electrocatalysts for CO2 conversion, but these materials also typically facilitate competing reactions, which compromises their efficiency.
Yasufumi Takahashi from Nano Life Science Institute (WPI-NanoLSI), Kanazawa University and colleagues have now identified a 2D metal dichalcogenide that can efficiently reduce CO2 to formic acid, a compound that not only occurs naturally but also is an intermediate product in chemical synthesis.
Takahashi and colleagues compared the catalytic performance of 2D sheets of disulfide (MoS2) and tin disulfide (SnS2). Both are 2D metal dichalcogenides, with the latter of particular interest because pure tin is a known catalyst for the production of formic acid. Electrochemical tests of these compounds revealed that with MoS2, instead of CO2 conversion, hydrogen evolution reaction (HER) was promoted. HER refers to a reaction yielding hydrogen, which can be useful when the production of hydrogen gas fuel is intended, but in the context of CO2 reduction it is an unwanted competing process. SnS2, on the other hand, showed good CO2 reduction activity and suppressed HER. The researchers also carried out electrochemical measurements for bulk SnS2 powder, which was found to have less catalytic CO2 reduction activity.
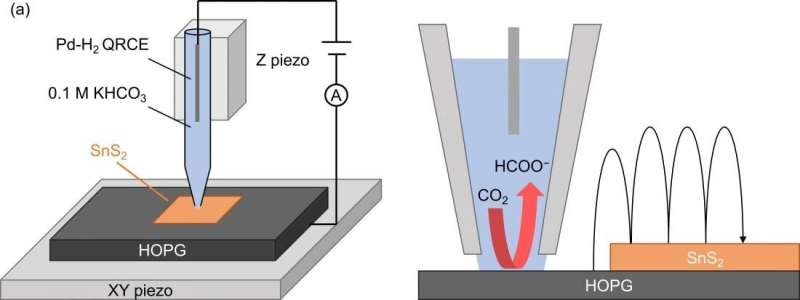
To understand where the catalytically active sites are in SnS2, and why the 2D material performs better than the bulk compound, the scientists applied a method called scanning electrochemical cell microscopy (SECCM). SECCM is used as a nanopipette to form the meniscus shape nanoscale electrochemical cell for the surface reactivity sensing probe on the sample. The measurements revealed that the whole surface of the SnS2 sheet is catalytically active, not only "terrace" or "edge" features in the structure. This also explains why 2D SnS2 has enhanced activity compared to bulk SnS2.
Calculations provided further insights into the chemical reactions at play. Specifically, the formation of formic acid was confirmed as an energetically favorable reaction pathway for when using 2D SnS2 as catalyst.
The results of Takahashi and colleagues signify an important step forward towards the use of 2D electrocatalysts in electrochemical CO2 reduction applications. They state, "These findings will provide a better understanding and design strategies for metal dichalcogenide-based 2D electrocatalysis for electrochemical CO2 reduction to produce hydrocarbons, alcohols, fatty acids and olefins without by-products."
Background: Two-dimensional metal dichalcogenides
Two-dimensional (2D) metal dichalcogenide sheets (or monolayers) are materials of the type MX2, with M a metal atom like molybdenum (Mo) or tin (Sn) and X a chalcogen atom like sulfur (S). The structure can be represented as a layer of X atoms on top of a layer of M atoms on top of a layer of X atoms again. 2D metal dichalcogenides belong to the so-called class of 2D materials (also including graphene), a reference to their extreme thinness. 2D materials typically have different physical properties than their bulk (3D) counterparts.
2D metal dichalcogenides have been studied for their hydrogen evolution reaction (HER) electrocatalytic activity, a chemical process yielding hydrogen. But now, Yasufumi Takahashi from Kanazawa University and colleagues have found that the 2D metal dichalcogenide SnS2 displays no HER catalytic activity; instead, it is a catalyst for the electrochemical reduction of carbon dioxide (CO2) to formic acid—an extremely relevant property in the context of strategies for reducing the global CO2 footprint.
More information: Yusuke Kawabe et al, 1T/1H-SnS2 Sheets for Electrochemical CO2 Reduction to Formate, ACS Nano (2023). DOI: 10.1021/acsnano.2c12627
Journal information: ACS Nano
Provided by Kanazawa University