This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study unveils theoretical principle that carbon-based catalysts promote electrochemical reactions
Carbon-based catalysts have garnered extensive attention over the past decades as an economical alternative to noble metal catalysts for renewable energy systems.
A recent study, affiliated with UNIST, has unveiled the theoretical principle that carbon-based catalysts promote electrochemical reactions. The key to this is that the presence of carbon defects—along with the structural flexibility and chemical reactions—are combined to enable the improvement of catalytic activity even without the need of precious metal catalysts, such as platinum (Pt).
The oxygen reduction reaction (ORR) is an important electrochemical reaction and has been widely applied in renewable energy applications, such as hydrogen fuel cells, water splitting, and metal–air batteries (MABs). Currently, commercial Pt-based catalysts (PBCs) are used extensively for this purpose due to their superior catalytic activity. However, the high price, facile CO poisoning, and low durability of Pt hamper the large-scale applications of PBCs, and thus noted the research team.
Carbon-based catalysts are being actively studied as a leading alternative material, and the efficiency and composition of catalysts are being steadily improved. However, the cause of carbon-based catalysts promoting electrochemical reactions is not clear, slowing the development of carbon-based catalysts.
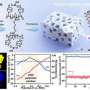
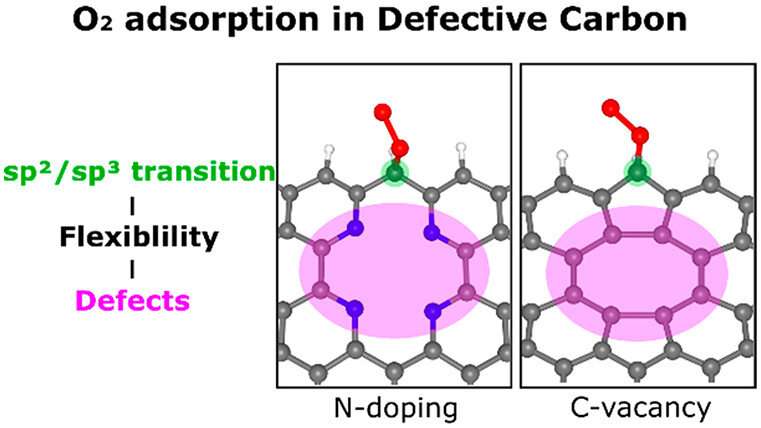
In this study, the research team demonstrated how two kinds of defects—carbon vacancies and pyridinic-N dopants, in metal-free carbon materials—enhance O2 adsorption, a critical reaction step for activating the ORR.
"Our calculations and structural analysis revealed that defects enable the neighboring edge carbon sites to adsorb O2 by enhancing the structural flexibility and, thus, lowering the energy barrier for the sp2/sp3 transition of the carbon atom," noted the research team. "Thus, the nonlocal structural environment is as critical as the direct interaction between the adsorption site and adsorbate in the chemical reaction."
Their findings further show that pyridinic-N has advantages over carbon vacancies in terms of structural stability. "Our study is based on numerous theoretical and experimental investigations of carbon materials and is consistent with catalytic experimental results," added the research team. "Moreover, our results can be extended to general chemical reactions in defective carbon materials and will spur research in defect engineering."
Their findings have been published in ACS Nano.
More information: Keunsu Choi et al, Theoretical Study of Oxygen Reduction Reaction Mechanism in Metal-Free Carbon Materials: Defects, Structural Flexibility, and Chemical Reaction, ACS Nano (2022). DOI: 10.1021/acsnano.2c05607
Journal information: ACS Nano