Seven years of carbon-based electrochemical catalysts: Where we are and where we need to go
The abundant carbon on Earth might offer a rich, renewable resource for clean, sustainable energy. The technology—called carbon-based electrochemical catalysis—that could make green energy conversion possible exists, according to an international collaboration investigating recent advancements, but is not ready for broad application.
While the catalysts have yet to hit the sweet spot of performance and cost-effectiveness needed for industrial deployment, the researchers said, there are clear pathways to advance the technology through the promise of carbon-based metal-free electrocatalysts (C-MFECs). The team published their review on December 15 in Nano Research Energy.
"It is imperative to develop sustainable and clear energy as well as related storage devices to alleviate the energy shortage and environmental pollution," said co-corresponding author Liming Dai, Scientia Professor, Australian Research Council Laureate and funding director of the Australian Carbon Materials Centre, School of Chemical Engineering, University of New South Wales.
"In this article, we provide a concise but critical review of recent progress in the development of rationally designed C-MFECs with high-performance activity sites for energy-related reactions and systems. We also discuss current challenges and future opportunities to provide forward-looking guidance for their potential application in various catalytic processes of practical significance."
Electrochemical catalysts typically accelerate a reaction at an electrode, which often require a metal. The metals that work best, such as platinum, are scarce and expensive. More common metals, such as iron and copper, are cheaper but less efficient in accelerating a full reaction.
According to co-corresponding author Chuangang Hu, corresponding author and a professor from the State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, C-MFECs offer a potential alternative to noble metal-based and transition metal-based electrocatalysts.
"Low-cost, high-activity and stable metal-free alternatives for renewable energy technologies are desperately desired," Hu said.
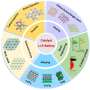
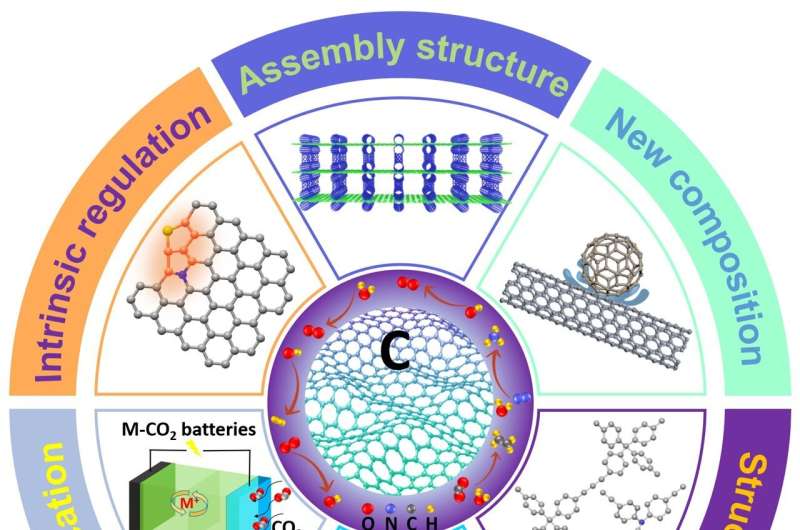
"After about a decade worldwide extensive research and development and with the availabilities of these newly developed strategies, C-MFECs show potential applications in renewable energy and environmental technologies of practical significance. Mainly since 2015, many strategies have been reported to improve electrochemical catalytic activity by designing C-MFECs through intrinsic catalytic structure adjustment and rational assembly."
In their assessment of the last seven years, roughly, of scientific literature, the researchers found that the most recent work includes how to control focus on structure design and regulation of intrinsic catalytic active sites, or how efficiently and effectively the catalyst causes the desired reaction. The recent work also includes advancements in construction of 3D assembly and composite structures and investigations into the mechanisms underlying C-MFECs.
"Recent years have witnessed tremendous progress in the field of C-MFECs," Dai said. "Rational design and regulation of the configuration and structure of C-MFECs could be used for tailoring advanced catalysts with desired properties and performance, which could make C-MFECs overtake metal-based catalysts in the race to the renewable energy technological marketplace."
To support the advancement of C-MFECs as a metal alternative for practical applications at a large scale, Dai said there is still an "urgent" need to develop efficient and controllable synthesis strategies. According to Dai and Hu, researchers should focus on overcoming key challenges to generate large-scale, reproducible C-MFECs with uniform and stable electrocatalytic active sites for specific reactions.
These barriers include developing better synthesis and precise control of C-MFECs' structure and properties; improving the characterization of the catalysts and their active sites to better inform theoretical modeling; developing multifunctional C-MFECs; and preparing C-MFECs for industrialization.
"Our goal is to provide a timely and concise, but critical review of recent progress in the development of C-MFECs as meaningful guidance for the design and synthesis of high-performance C-MFECs." Hu said.
More information: Jixin Yan et al, Recent progress in carbon-based electrochemical catalysts: From structure design to potential applications, Nano Research Energy (2022). DOI: 10.26599/NRE.2023.9120047
Provided by Tsinghua University Press