This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Building better catalysts to close the carbon dioxide loop
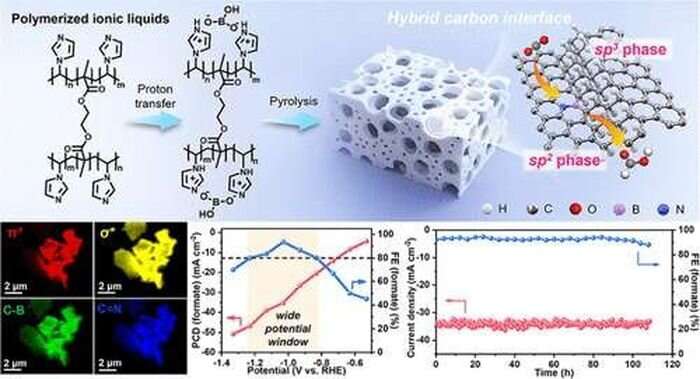
The best way to stave off the worst effects of climate change is to reduce CO2 emissions around the world. And one way to do that, says Zhongwei Chen, a professor in the Department of Chemical Engineering at the University of Waterloo, is to capture the CO2 and convert it into other useful chemicals, such as methanol and methane for fuels.
Stopping emissions at the source, and further reducing future ones by replacing CO2-producing fuels with cleaner ones "…is a way to close the circle," Chen says.
In order to turn CO2 into methanol, you need a catalyst to jump-start the electrochemical reaction. Traditionally, these catalysts have either been made out of precious metals like gold or palladium, or base metals like copper or tin. However, they are expensive and break down easily, hindering large-scale implementation.
"Right now we can't meet industrial requirements," says Chen. "So we are trying to design catalysts with better activity, selectivity, and durability."
Chen and his team are focused on low-cost metal and metal-free catalysts. The metal-free catalysts, made from carbon, are cheaper and more durable but tend to have lower catalytic activity than metal ones. So the team tweaked the chemical composition and physical design of the catalyst to optimize its efficiency, combining the materials science of the catalyst design with the engineering of the electrode and reactor to improve the activity of the whole system.
"We want to make it as small as we can, but not too small to be a practical application," says Chen. They combined nanometer-scale active sites within a micrometer-scale particle—like bubbles in a tiny sponge—to create a catalyst with a huge number of active sites in a particle that is easy and practical to fabricate.
The powerful light beams and expert technical teams at the Canadian Light Source (CLS) at the University of Saskatchewan were instrumental in helping to design an efficient catalyst, says Chen. "The advanced facilities at CLS are critical in helping us understand what was going on during the reaction, so we can continue to design and improve the next generation of catalysts."
The paper is published in the journal ACS Catalysis.
More information: Zhen Zhang et al, Steering Carbon Hybridization State in Carbon-Based Metal-free Catalysts for Selective and Durable CO2 Electroreduction, ACS Catalysis (2022). DOI: 10.1021/acscatal.2c03055
Journal information: ACS Catalysis
Provided by Canadian Light Source