This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
A panoramic view on lithium-mediated electrochemical dinitrogen reduction reaction
The Haber-Bosch process is the industrial approach for NH3 production today, which must be operated at energy-intensive high temperatures and pressures. The reduction of dinitrogen (N2) by electrocatalysis offers an alternative way for NH3 production at ambient conditions and a variety of electrocatalysts have been studied over the past few years.
However, even the best catalytic system reported could only get unsatisfied performance (such as the selectivity and production rate of NH3) due to the inertness of N2. The lithium-mediated N2 reduction reaction (Li-eN2RR) has been found to be a promising route to promote electrochemical NH3 synthesis.
In this regard, a group of researchers has summarized the reaction mechanisms, the catalysts developed, and the electrolytes involved based on the most recent research progress of Li-eN2RR. They also point out the challenges and possible resolving strategies in the Li-eN2RR.
This could provide a panoramic view of the related field and facilitate the development of Li-eN2RR for green NH3 production. Sun et al. published their review in Industrial Chemistry & Materials.
"The electrocatalytic reduction of N2 for the production of NH3 has been the subject of extensive research, which has been comprehensively reviewed by numerous scholars with expertise in the field," said corresponding author Zhenyu Sun, a professor at Beijing University of Chemical Technology.
"These reviews provide detailed insights into the catalytic performance and mechanisms involved, which can be of great assistance to researchers. However, the NH3 production rate is still low, far behind the requirement of industrial application. The lithium-mediated N2 reduction reaction (Li-eN2RR) holds great potential for the production of NH3 under ambient conditions."
"Therefore, we provide this comprehensive overview to summarize the recent progress of Li-eN2RR. This review demonstrates the basic aspects of Li-eN2RR including the common composition of electrolytes, suppression of hydrogen evolution, reaction mechanism, the reported electrocatalysts, and the challenges and prospects, which can be helpful to new individuals in this field as well as serve as a valuable resource for researchers, policymakers, and industry professionals alike."
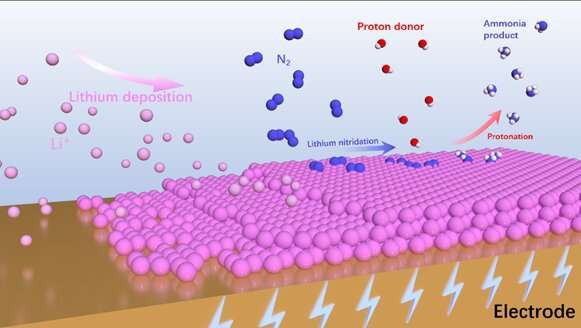
The rate-determining step in the synthesis of NH3 is often the adsorption and activation of N2. However, due to the inertness of N2, it is extremely hard to break the N≡N triple bond. In the Li-eN2RR process, Li can be used as a mediator to fix N2 as Li3N, which subsequently converts to NH3.
"There have been different viewpoints on the reaction mechanism of Li-eN2RR. We provide detailed analysis of the reported mechanisms," Sun said.
Three different mechanisms, including chemical N2 splitting and chemical protonation, N2 activation and protonation through an associative mechanism, and chemical N2 splitting and electrochemical protonation were introduced with their respective reaction steps.
The section about the reaction mechanisms of Li-eN2RR demonstrates the different routes of N2 activation and hydrogenation to NH3 production. "Through the different mechanisms, we can have a comprehensive understanding of Li-eN2RR, which can guide the catalytic system design."
The rational design of electrocatalysts is crucial for Li-eN2RR. There have been some strategies for the engineering of the electrodes to get enhanced performance.
"The electrocatalysts together with their design strategies were categorized based on the metal species involved," Sun said.
"Different kinds of electrocatalysts including noble metal catalysts such as Ru, Ag, Au; nonprecious metal catalysts such as copper-based materials, molybdenum, lithium-liquid alloy-salt, stainless steel cloth; and non-metal catalysts such as carbon-based materials were comprehensively overviewed. It was discussed in detail about their performance and the catalytic active sites in Li-eN2RR, which may enlighten the design of the future catalysts towards efficient NH3 production."
Another important part of Li-eN2RR is the electrolytes. According to Zhenyu Sun, more attention should be given to the study of electrolytes in electrocatalytic N2 reduction considering the significance of electrolytes for the reaction and for N2 dissolution.
Some Li-containing electrolytes have been developed for Li-eN2RR, yet more efforts still need to be devoted to further improving the NH3 yield. This requires the synergistic work between the electrolytes and the electrocatalysts as well as the study of their interface.
"The primary objective of this review is to provide readers with a clear understanding of the current research progress of Li-eN2RR, which is still in its early stage yet promising for electrocatalytic NH3 production. We also highlight the challenges and propose strategies to overcome them. We hope it is helpful to promote the development of Li-eN2RR towards green NH3 production," Sun said.
More information: Muhammad Saqlain Iqbal et al, Lithium-mediated electrochemical dinitrogen reduction reaction, Industrial Chemistry & Materials (2023). DOI: 10.1039/D3IM00006K
Provided by Industrial Chemistry & Materials