This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Reviewing and evaluating recent electrochemical carbon dioxide reduction with ionic liquids
The increasing CO2 emission as the chief culprit causing many environmental problems could be addressed via electrochemical CO2 reduction (CO2R) to the added-value carbon-based chemicals. Due to the unique advantages, ionic liquids (ILs) have been widely studied to promote CO2R as electrolytes and co-catalysts.
Among the potential products of CO2R, those only containing one carbon atom, named C1 products, including CO, CH3OH, CH4, and syngas, are easier to achieve than others. In the last dozen years, numerous related experimental studies and reviews have been reported to promote the development of CO2R-to-C1 products, and rapid progress has been achieved in these recent years.
However, to the best of our knowledge, no work has been conducted to discuss and systematically compare the economic benefits of different C1 products (CO, CH3OH, CH4, H2/CO(1:1) and H2/CO(2:1)) focusing on the IL-based electrolyte systems, as well as analyzing environmental impacts, to give guidance for realizing the commercialization of CO2R technology in the near future.
Herein, a team of scientists summarized and updated the research progress in the CO2R-to-C1 products based on the IL-based electrolytes, and comprehensively evaluated the economic benefit and environmental influence for the state-of-the-art and future potential technologies, respectively. Their work was published in Industrial Chemistry & Materials.
"As the rapid development of CO2R with ILs-based electrolyte in the lab, it is necessary to have a clear insight on their commercial value when scale-up it to an industrial scale," said Xiaoyan Ji, a professor at Luleå University of Technology.
"In this review, we summarized the experimental achievements of CO2R-to-C1 products using IL-based electrolyte, evaluated their performances from both economic and environmental aspects based the state-of-the-art technology and those with improved performance in the near future, and identified their potential for commercialization. We also put forward the strategies to boost the performance and profits for CO2R-to-C1 product with ILs as the electrolyte in the future."
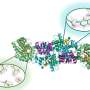
CO2R is one of the most promising methods to realize the conversion of CO2 to value-added chemicals because of its mild conditions, as well as its easy and flexible controllability. Besides, its driving force can be integrated with renewable sources, such as solar, wind, and hydropower.
There are three main parameters to evaluate the performance of CO2R, including current density, Faradaic efficiency (FE), and cell voltage, which can be improved through designing and optimizing electrocatalysts and electrolytes.
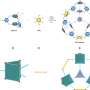
ILs, with their tunable structures and properties, wide electrochemical windows, and high electrical conductivities, can provide a low overpotential and high current density, and improve the product selectivity for CO2R. Significantly, ILs can effectively inhibit the hydrogen evolution reaction (HER), which is a competitive reaction of CO2R.
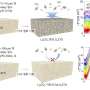
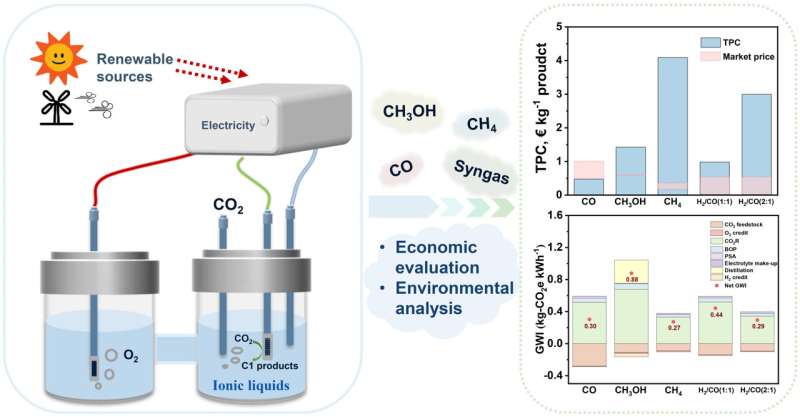
"CO is the only profitable product among the studied C1 products, while the total production costs (TPC) of other products are too high to be profitable, especially for CH4 and H2/CO(2:1)," Ji said.
"This phenomenon is consistent with the performance of CO2R for each product. The current density and FE of CO are as high as 182.2 mA cm-2 and 99.7%, respectively. However, for CH4 and H2/CO(2:1), the current densities are as low as 25.6 and 11.4 mA cm-2, respectively. Additionally, with the improved performance of CO2R, CH3OH and H2/CO(1:1) can be profitable in the near future. While it is difficult for CH4 and H2/CO(2:1) to be profitable even under the most ideal scenario, partly due to the low market price. On the other side, the formation of CH4 requires transferring the largest number of electrons (8e-) among the studied C1 products."
"CO2R-to-CH4 is the most environmentally friendly pathway compared to others," added Xiangping Zhang, a professor at the Institute of Process Engineering, Chinese Academy of Sciences. "While, considering both economic and environmental aspects, CO is the most attractive product. For other C1 products, further improvement of CO2R or the development of more advanced electrolyzers are required to realize their commercial value," Zhang said.
Furthermore, ILs should be further exploited in the future CO2R as follows: (1) the adjustable feature of ILs in the structure and properties provides unique advantages and feasibilities for designing more efficient and suitable electrolytes of CO2R; (2) the capability of ILs to dissolve a variety of solvents and electrolytes can integrate other solvents and electrolytes, further improving the performance of CO2R; (3) the cleaner ILs can be designed and synthesized applying into CO2R to mitigate the environmental burden; (4) except as electrolytes, ILs can also be the co-catalyst or modifier for the catalyst exhibiting superior performance.
"In this review, our main goal is to provide readers with intuitionistic insight on the commercial potential of CO2R-to-C1 products with ILs as the electrolyte based on the state-of-the-art and future scenarios from both economic and environmental aspects," said Ji.
More information: Yangshuo Li et al, Electrochemical CO2 reduction with ionic liquids: review and evaluation, Industrial Chemistry & Materials (2023). DOI: 10.1039/D2IM00055E
Provided by Industrial Chemistry & Materials