This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Investigation of NiFe-based catalysts for water oxidation in different pH electrolytes
Renewable electricity driven water splitting offers a green and sustainable way to produce hydrogen (H2). The key to improving the water splitting efficiency is an efficient electrocatalyst. Non-noble nickel iron (NiFe)-based electrocatalysts are among the best catalysts for oxygen evolution reaction (OER) in alkaline electrolytes. However, they show much lower activity in neutral pH conditions, which limits their application in seawater splitting and CO2 reduction.
Despite the fact that many forms of NiFe-based electrocatalysts have been reported, they have poor OER performance. To obtain a fundamental understanding of the mechanisms and functionalities of NiFe electrocatalysts, researchers must track their compositional and structural changes during the OER process.
Recently, research teams led by Prof. Jingshan Luo from Nankai University and Prof. Junhu Wang from Dalian Institute of Chemical Physics, Chinese Academy of Sciences reported their research on the OER mechanism of NiFe based catalysts in different pH electrolytes. This work presents a systematic electrochemical and operando spectroscopy study of NiFe LDH for OER in different pH electrolytes. The study is published in Chinese Journal of Catalysis.
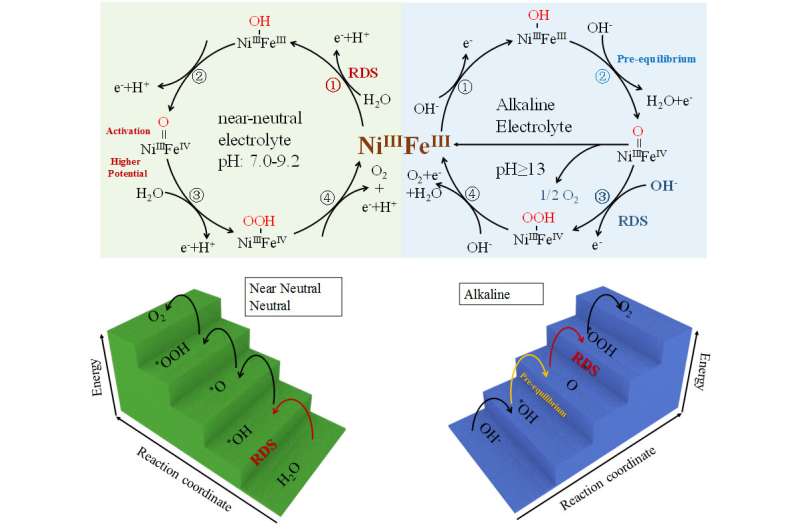
The NiFe LDH catalysts showed a clear pH-dependent OER activity. Operando/ex-situ Raman spectroscopy indicated the formation of Ni3+ species requires higher potential in neutral and near-neutral conditions than in the alkaline electrolyte. The operando 57Fe Mössbauer spectra results also confirmed that Fe4+ species are difficult to form or generated at the more positive potential in neutral or near neutral conditions, compared to NiFe LDH in alkaline conditions.
In addition to the different feasibility for forming high-valent Ni3+ and Fe4+ species, the rate-determining step (RDS) of NiFe LDH for OER was also different in different pH conditions. In alkaline conditions, the barrier for the *OH adsorption and formation of *O is reduced, and the transformation of *O to *OOH is the RDS, leading to a high OER performance. While in a neutral medium, the *OH adsorption is the RDS.
The team's results provide new insights for understanding the OER mechanism, and will promote the design of highly efficient electrocatalysts for water oxidation.
More information: Qixian Xie et al, Investigation of nickel iron layered double hydroxide for water oxidation in different pH electrolytes, Chinese Journal of Catalysis (2022). DOI: 10.1016/S1872-2067(22)64190-1
Provided by Chinese Academy of Sciences