This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Breaking inert bonds: Multicomponent catalysts pave the way for green chemistry and green carbon science
The chemical industry has played a significant role in the development of society, but its impact on the environment has become a growing concern. Green chemistry and chemical engineering have opened up possibilities for sustainability through the transformation of renewable feedstocks into environmentally friendly chemicals. However, the inert bonds in molecules such as CO2 and N2 present challenges to their activation and conversion.
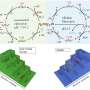
Electrochemical conversion provides a promising carbon-neutral route to upgrading green chemical sources with inert bonds to chemicals and fuels under ambient conditions. Multicomponent electrocatalysts have advantages over monocomponent catalysts, such as better stability, increased activity, and expanded reaction processes.
Multicomponent electrocatalysts offer a promising solution to the challenge of sustainability in the chemical industry. A group of researchers published their review on Industrial Chemistry & Materials.
"The chemical industry has played a crucial role in society's historical evolution, but it also presents emerging environmental concerns and skyrocketing CO2 emissions," said corresponding author Buxing Han, the member of the Chinese Academy of Sciences, a professor at Institute of Chemistry, Chinese Academy of Sciences (ICCAS).
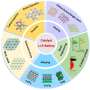
"We were motivated to explore the possibilities of green chemistry and chemical engineering to transform renewable feedstocks, such as CO2 and NOx, into environmentally friendly chemicals, including syngas, hydrocarbons, oxygenates, and ammonia."
"However, these inert bonds, such as the C=O bond in CO2, pose challenges to their activation and conversion. We wanted to explore electrochemical conversion as a universal carbon-neutral route to efficiently upgrade green chemical sources with inert bonds to chemicals and fuels under ambient conditions harnessing clean energy," said co-corresponding author Prof. Xiaofu Sun, ICCAS.
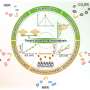
"Multicomponent electrocatalysts offer advantages over monocomponent catalysts in terms of stability, activity, and reaction processes. So, we explored the use of multicomponent catalysts in the electroreduction of small molecules such as CO2, N2, and NOx. We developed three models for multicomponent catalysts: Type I, Type II, and Type III, which we discuss in our paper."
Type I involves a non-catalytic active component that can activate or protect another catalytic component. Type II involves all catalytic components providing active intermediates for electrochemical conversion. Type III involves one component providing the substrate for the other through conversion or adsorption.
"Each of these models has its own advantages and disadvantages, depending on the specific reaction and catalyst. We explored the use of these models in our paper to show their effectiveness in the electroreduction of small molecules," Han said. "And we also discussed future directions for applying multicomponent electrocatalysts in the industrial utilization of renewable chemical sources through highly efficient activation and conversion of inert bonds."
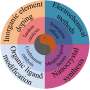
What are the key challenges that need to be addressed in the development and utilization of multicomponent electrocatalysts for the activation and conversion of renewable chemical sources? "One key challenge is improving the selectivity and efficiency of the electrocatalysts, as well as increasing their stability and activity," Sun said. "Another challenge is understanding the fundamental mechanisms of the electroreduction reactions and how they are influenced by the multicomponent catalysts."
"More importantly, there is a need for further research and development to scale up and integrate these electrochemical processes into industrial applications. Many promising research projects are undergoing in our lab," Han said.
More information: Shunhan Jia et al, Multicomponent catalyst design for CO2/N2/NOx electroreduction, Industrial Chemistry & Materials (2023). DOI: 10.1039/D2IM00056C
Provided by Industrial Chemistry & Materials