Examining the theories that guide the rational design of electrocatalysts
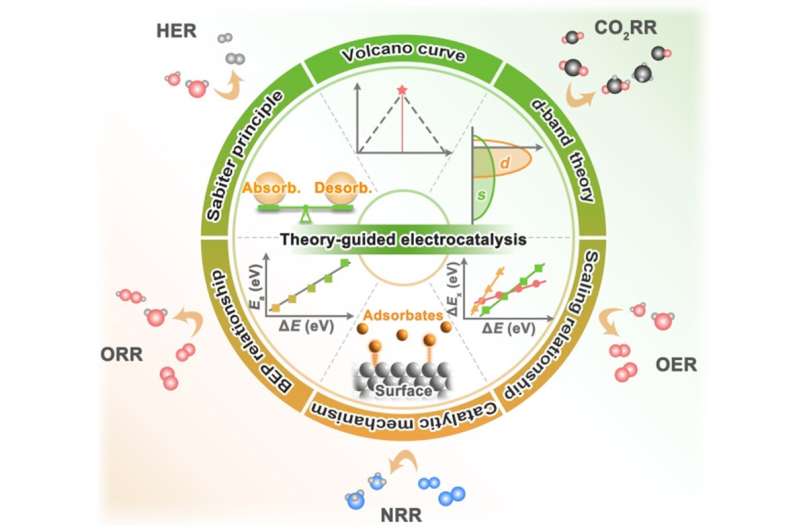
Electrocatalysis plays a crucial role in many clean energy conversion technologies, and it is able to couple with renewable energy generation systems such as photovoltaic, wind turbines and hydropower to solve future global energy problems and climate crises.
Some important electrochemical conversion processes, including hydrogen evolution reaction (HER), oxygen evolution reaction (OER), oxygen reduction reaction (ORR), nitrogen reduction reaction (NRR) and carbon dioxide reduction reaction (CO2RR), have attracted extensive research interest.
These electrochemical reactions are able to convert abundant natural resources into important chemicals (hydrogen, ammonia and organics) under mild conditions. However, these electrochemical reactions are kinetically sluggish and require high performance electrocatalysts to increase reaction efficiency and reduce additional energy consumption.
The current electrocatalysts have deficiencies in activity, stability and selectivity, and highly dependent on noble metals. There is an urgent need to develop new electrocatalysts.
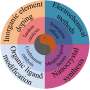
The research and development of traditional electrocatalysts relies on trial-and-error approaches, which is time-consuming and costly. In the past 20 years, the development of new materials based on theoretical guidance has become more advanced design ideas for electrocatalysts, which mainly benefits from the establishment of important basic theories, activity descriptors and catalyst mechanisms, and the maturity of computational chemistry in the field of electrochemistry.
These advances reveal the structural-activity law of electrocatalyst and accelerate the development process of electrocatalyst.
Recently, a research team led by Prof. Xiaoxin Zou and Prof. Hui Chen from Jilin University, China has combed the development process of electrocatalyst guiding theory. Starting from the early qualitative Sabiter principle, with the aid of computational chemistry, Volcano curves, Brønsted-Evans-Polanyi relations and scaling relations have become important theories for quantitative description of catalyst activity.
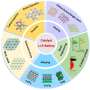
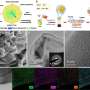
The theory of d-band center and the regulation method of d-band (strain effect and ligand effect) are described in detail. For important electrocatalytic reactions (HER,OER, ORR, NRR and CO2RR), the mainstream reaction mechanisms, theoretical research progress and descriptors were summarized by combining the latest high-throughput screening and machine learning methods.
Challenges and perspectives on the development of highly performance electrocatalysts based on theory guiding are also discussed. The results were published in Chinese Journal of Catalysis.
More information: Mingcheng Zhang et al, Theory-guided electrocatalyst engineering: From mechanism analysis to structural design, Chinese Journal of Catalysis (2022). DOI: 10.1016/S1872-2067(22)64103-2
Provided by Chinese Academy of Sciences