This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Chemical functionalized noble metal nanocrystals for electrocatalysis
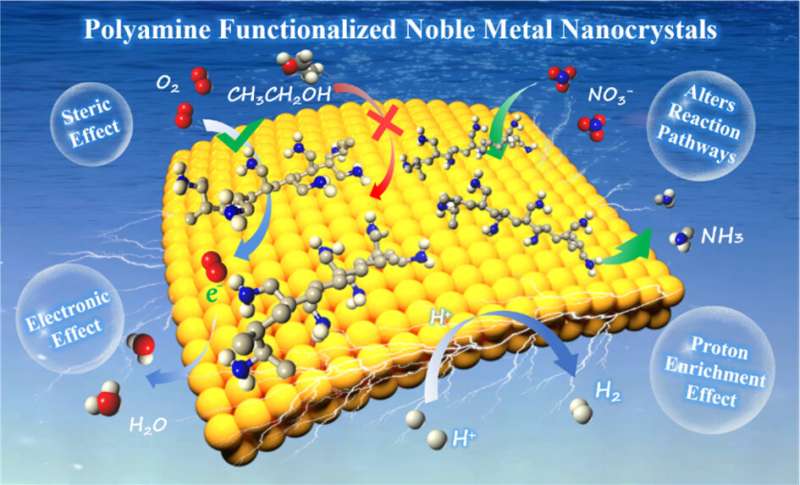
Electrocatalysis is an interface-dominated process, in which the activity of the catalyst highly relates to the adsorption/desorption behaviors of the reactants/intermediates/products on the active sites. From the perspective of catalyst design, the chemical functionalization on noble metal surfaces will inevitably affect the reaction process, which is considered to be one of the effective strategies to tune the electrocatalytic performance of noble metal nanocrystals.
Recently, a research team led by Prof. Yu Chen from Shaanxi Normal University, China published a paper in the field of noble metal electrocatalysis. Their paper summarizes the synthesis methods of polyamine (PAM) functionalized noble metal nano-electrocatalysts and their applications in electrocatalytic reactions, and presents the research progress, current deficiencies, challenges and future prospects of chemically functionalized noble metal electrocatalysts, which were published in Chinese Journal of Catalysis.
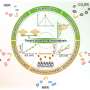
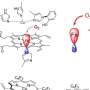
The formation mechanism of PAM molecule functionalized noble metal nanocrystals first is discussed. The authors explain that PAM has a large number of amino groups (−NH2) and/or imino groups (−NH−), in which the lone pair of electrons on the nitrogen atom has a strong coordination ability. In the hydrothermal reaction, PAM can well interact with PtII, RhIII, PdII and AgI to form complexes, which transforms the growth process of noble metal nanocrystals from thermodynamic control to kinetics control.
Under kinetic control, the final shape of noble metal nanocrystals no longer tends to form nanospheres with minimal surface free energy, and various anisotropic nanostructures will be obtained based on the reaction conditions, such as nanocubes, nanowires, nanosheets and nanonetworks.
The PAM functionalized electrocatalysts are applied in some important electrochemical reactions such as hydrogen precipitation reaction (HER) and oxygen reduction reaction (ORR), which generally reveal enhanced electroactivity. Typically, a large amount of −NH2 and −NH− in PAM will be protonated to form −NH3+ and −NH2+ in acidic or neutral media, which will directly lead to the increase of the surface proton concentration of PAM-functionalized noble metal nanocrystals.
For the proton-coupled electrocatalytic reactions, such as HER and ORR, PAM-functionalized noble metal nanocrystals exhibit lower reaction overpotentials and higher catalytic efficiency due to the interfacial proton enrichment. In addition, the effects of PAM functionalization (such as electronic effect, steric hindrance effect, group effect) on catalyst activity and selectivity are highlighted.
Finally, shortcomings, challenges and perspectives in this promising emerging research field are briefly summarized. This work aims to stimulate deeper attention to surfaces/interfaces functionalization and catalysis, increase investment in surfaces/interfaces functionalization research, and change our future renewable energy production and environmental technologies related to electrocatalysis.
More information: Qi Xue et al, Chemical functionalized noble metal nanocrystals for electrocatalysis, Chinese Journal of Catalysis (2023). DOI: 10.1016/S1872-2067(22)64186-X
Provided by Chinese Academy of Sciences