This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers synthesize N and P co-doping catalysts
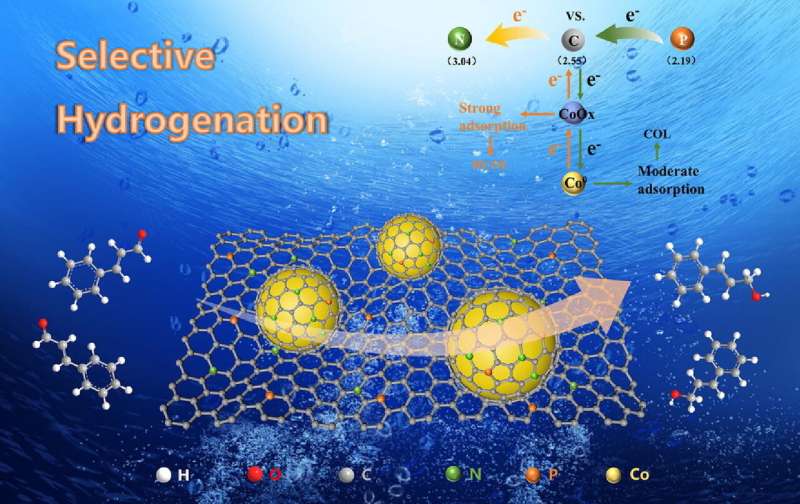
Recently, the team from Institute of Solid State Physics, Hefei Institutes of Physical Science of the Chinese Academy of Sciences, made a breakthrough in the research of non-metallic heteroatom doping to regulate catalytic performance. The researchers synthesized N and P co-doping carbon-coated cobalt-based catalysts (Co@NPC) and explored the effect of heteroatom doping in the catalyst on the selective hydrogenation of cinnamaldehyde. The study was published in the Journal of Catalysis.
As a potential material in the field of catalysis, heterogeneous catalyst of metal nanoparticles supported on non-metallic atoms doped carbon supports demonstrate great catalytic activity in the catalytic hydrogenation reaction. Compared with metal atoms, non-metal atoms possess greater electronegativity. Therefore, the introduction of non-metal heteroatoms can effectively modulate the electronic structure and chemical properties of the materials and produce rich active sites to improve catalytic performance.
In this study, researchers prepared Co@NPC and applied it in the aqueous hydrogenation reaction of cinnamaldehyde. The catalyst was coated by the carbon layer during the synthesis process (hydrothermal and calcination). Once the carbon formed a coating layer, it could prevent the metal from sintering, leaching and growing up. This structure displayed highly dispersed abundant active sites, thus contributing to the excellent activity catalyst performance.
Co@NPC exhibited excellent performance in reaction. It can achieve 92.1% conversion of cinnamaldehyde and 79.7% selectivity of cinnamaldehyde respectively under the reaction conditions of 80℃, 0.5 MPa hydrogen pressure and 5 h. Compared with the catalyst Co@C without doping, N, P co-doping can significantly improve the catalytic performance and control the activity and selectivity of the reaction by adjusting the doping amount.
In addition, the researchers proved that P doping could improve the selectivity of cinnamyl alcohol, while N doping was closely related to the catalytic activity of the catalyst. Density functional theory (DFT) calculation showed that the electronegativity difference between N, P and C atoms led to the interaction between electrons and changed the ratio of Co and CoO in the catalyst, thereby changing the adsorption strength of cinnamaldehyde on the surface of the catalyst. The reaction products can be adjusted according to the difference of adsorption energy.
This study showed that doping of non-metallic atoms made it possible to regulate the electronic structure of catalyst, which provides a new strategy for the design of catalyst for selective hydrogenation of biomass derivatives.
More information: Yue Shen et al, Geometric and electronic effects of Co@NPC catalyst in chemoselective hydrogenation: Tunable activity and selectivity via N,P co-doping, Journal of Catalysis (2023). DOI: 10.1016/j.jcat.2023.03.013
Provided by Chinese Academy of Sciences