This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Computer simulations deepen our understanding of how drugs get into the blood
There is a need for new drugs. For example, many of the antibiotics that we have been using for a long time are becoming less effective. Chemists and pharmaceutical scientists are frantically searching for new active substances, especially those that can penetrate cell membranes, as these are the only ones that patients can take orally in the form of a tablet or syrup.
Only active ingredients pass through the intestinal wall in the small intestine and enter the bloodstream to reach the affected area in the body. For active ingredients that cannot penetrate the cell membrane, physicians have no choice but to inject them directly into the bloodstream.
Large molecules with potential
That is why researchers are trying to understand which molecules can penetrate cell membranes and how exactly they do this. For one important and promising class of substances—cyclic peptides—chemists at ETH Zurich have now decoded additional details of the relevant mechanism. "The more we know about this mechanism and the properties a molecule must have, the earlier and more effectively researchers can take this into account when developing new drugs," says Sereina Riniker, a professor in the Department of Chemistry and Applied Biosciences. She led the study, which has now been published in the Journal of Medicinal Chemistry.
Cyclic peptides are ring-shaped molecules that are much larger than the small molecules that make up the majority of today's drugs. In some areas of application, however, chemists and pharmaceutical scientists are coming up against their limits with small molecules, which is why they are turning to larger molecules like the cyclic peptides. This substance class includes many pharmaceutically active natural substances, such as cyclosporine, an immunosuppressant that for decades has been used after organ transplants, and many antibiotics.
Possible only with computer modeling
Using computer modeling and a lot of supercomputer power, Riniker and her colleagues were able to elucidate how cyclic peptides similar to cyclosporine cross a membrane. "Only modeling allows us such detailed, high-resolution insights, as there are no experiments that would let us observe an individual molecule crossing a membrane," Riniker says.
To understand the mechanism, one must know how cyclic peptides are structured: they consist of a central ring structure to which side chains are attached. The molecules are flexible and can dynamically change their structure to adapt to their environment.
Dance through the cell membrane
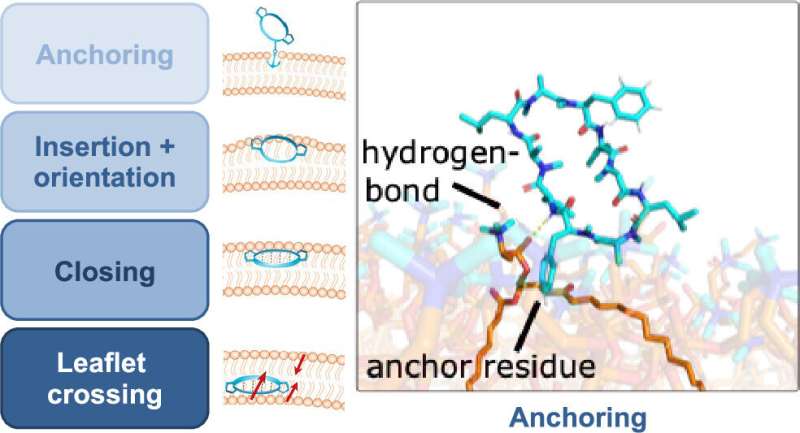
Riniker's simulations reveal in detail how a cyclic peptide penetrates the membrane: First, the molecule anchor itself to the membrane's surface, before penetrating it perpendicular to the membrane. It then changes its three-dimensional shape while passing through, rotating once about its longitudinal axis before reaching the other side of the membrane, where it exits again.
These changes in shape have to do with the different environments the molecule experiences as it moves through the membrane: The body consists largely of water. Both inside and outside of cells, biochemical molecules are mostly present in aqueous solution. Cell membranes, on the other hand, are made up of fatty acids, so water-repellent conditions prevail within them. "To enable it to cross the membrane, the cyclic peptide changes its three-dimensional shape to briefly become as hydrophobic as possible," Riniker explains.
Changing molecular side chains
For the present study, the researchers investigated eight different cyclic peptides. These are model peptides with no medicinal effect—scientists at pharmaceutical giant Novartis developed them for basic research, which is why Riniker also collaborated with Novartis researchers for this study.
The new findings can now be used in discovering cyclic peptides as new drug candidates. However, Riniker points out a certain trade-off: there are side chains that provide ideal conditions for cyclic peptides to anchor to the membrane surface, but that make it difficult for the peptides to cross the membrane. This new knowledge helps researchers to give advance thought to which side chains they want to use and where on the molecule they are most helpful.
All of this could speed up drug discovery and development by ensuring right from the outset that researchers are investigating potential active ingredients that can eventually be taken as a tablet.
More information: Stephanie M. Linker et al, Lessons for Oral Bioavailability: How Conformationally Flexible Cyclic Peptides Enter and Cross Lipid Membranes, Journal of Medicinal Chemistry (2023). DOI: 10.1021/acs.jmedchem.2c01837
Journal information: Journal of Medicinal Chemistry
Provided by ETH Zurich