This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Decoding the mechanisms behind the assembly of BAR proteins that dictate cell curvature
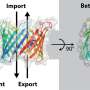
Cell membranes play a critical role by serving as containment units and separating the inner cellular space from the extracellular environment. Proteins with distinct functional units play a key role in facilitating protein-membrane interactions. For instance, Bin-Amphiphysin-Rvs (BAR) domain proteins are involved in regulating cell membrane curvature. This physical bending of cell membranes helps cells carry out various biologically important processes such as endocytosis and cell motility. Although BAR proteins drive membrane curvature by assembling into highly ordered oligomeric units, the underlying mechanism regulating this phenomenon remains largely unknown.
Now, a study by researchers from Japan has revealed the mechanism that drives the oligomeric assembly of a BAR-domain-containing protein on membrane surfaces. The study, published in the journal Science Advances, was led by Shiro Suetsugu, Wan Nurul Izzati Wan Mohamad Noor, and Nhung Thi Hong Nguyen from Nara Institute of Science and Technology (NAIST).
Suetsugu says, "The relatively small number of oligomeric BAR domains on narrow membrane tubules makes it difficult to analyze their assembly. We thus used fluorescence resonance energy transfer monitoring to analyze the oligomeric assembly of F-BAR-containing GAS7 protein, because oligomeric GAS7 assembles into larger than the others."
To elucidate the mechanism involved in the assembly of GAS7 on membrane surfaces, the researchers employed a technique called fluorescence resonance energy transfer (FRET). In this method, the researchers labeled GAS7b units with fluorescent protein tags to monitor the magnitude and timing of GAS7 assembly.
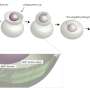
The observation of fluorescence emission indicated that GAS7 assembly on lipid membrane surfaces was a rapid process and started within seconds. This process was enhanced by the presence of several proteins, including the Wiskott-Aldrich syndrome protein (WASP)/N-WASP, WISH, Nck, the activated small GTPase Cdc42, and a membrane-anchored phagocytic receptor.
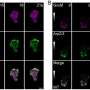
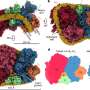
The assembly of GAS7 on the membrane was also examined by microscope, using giant membrane vesicles. The protein should bind to the membrane uniformly if it does not oligomerize but GAS7 clearly accumulated at the part of the membrane, demonstrating the oligomeric assembly by the presence of those proteins.
The team further examined the role of WASP in GAS7 assembly. WASP undergoes mutations in patients with Wiskott-Aldrich syndrome, which is associated with various immunological disorders. In this regard, the researchers saw that the regulated GAS7 assembly was abolished by the WASP mutations both in vitro as well as during phagocytosis (the cell-membrane-mediated engulfment of large particles).
The latter, according to the researchers, was striking, because GAS7 is known to be involved in phagocytosis. Therefore, the analyses provided an explanation for the defective phagocytosis seen in macrophages from patients with Wiskott-Aldrich syndrome.
In conclusion, WASP, Cdc42, and the other proteins that commonly bind to the BAR domain superfamily proteins promote GAS7 assembly on lipid membranes. Moreover, BAR domain assembly on membrane surfaces serves as a "scaffold" or platform for the binding of other proteins, which further facilitates protein signaling beneath the surface.
Summarizing the results, Suetsugu concludes, "Since WASP protein commonly binds to the BAR superfamily of proteins, the mechanism of assembly observed here is likely to function for other BAR proteins as well. We believe that our study provides breakthrough information for studies on cellular shape formation and protein condensate studies."
More information: Wan Nurul Izzati Wan Mohamad Noor et al, Small GTPase Cdc42, WASP, and scaffold proteins for higher order assembly of the F-BAR domain protein, Science Advances (2023). DOI: 10.1126/sciadv.adf5143. www.science.org/doi/10.1126/sciadv.adf5143
Journal information: Science Advances
Provided by Nara Institute of Science and Technology