This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
MXene interlayers: New proton hydration structure determined
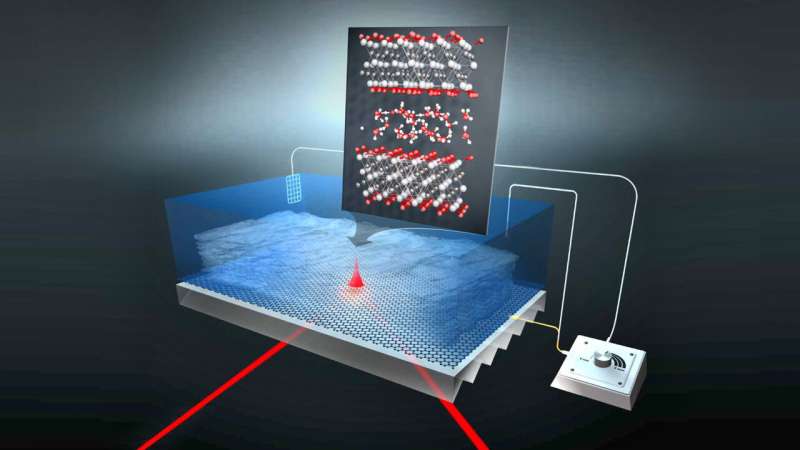
MXenes are able to store large amounts of electrical energy like batteries and to charge and discharge rather quickly like a supercapacitor. They are thus a very interesting class of materials for energy storage. The material is structured like a kind of pastry, with the MXene layers separated by thin water films. A team at HZB has now investigated how protons migrate in the water films confined between the layers of the material and enable charge transport. Their results have been published in the journal Nature Communications and may accelerate the optimization of these kinds of energy storage materials.
One of the biggest challenges for a climate-neutral energy supply is the storage of electrical energy. Conventional batteries can hold large amounts of energy, but the charging and discharging processes take time. Supercapacitors, on the other hand, charge very quickly but are limited in the amount of stored energy. Only in the last few years has a new class of materials been discussed that combines the advantages of batteries with those of supercapacitors, named pseudocapacitors.
Promising materials: Pseudocapacitors
Among pseudocapacitive materials, so-called MXenes consisting of a large family of 2D transition metal carbides and nitrides appear particularly promising. Their structure resembles a puff pastry, with the individual layers separated by a thin film of water that enables the transport of charges.
Titanium carbide MXenes, especially, are conductive and their layered structure combined with highly negatively-charged hydrophilic surfaces offers a unique material in which positively charged ions such as protons can diffuse very efficiently. The MXenes used in this study were synthesized in the group of Prof. Yury Gogotsi in Drexel University.
Over the last years, this property has been used to store and release energy from protons at unprecedented rates in acidic environment. It remains though unclear if the charges are mostly stored based on proton adsorption at the MXene surface or through desolation of proton in the MXene interlayer.
Due to its two-dimensional geometry, the 2-3 layer thick water film trapped between the MXene layers is expected to solvate protons differently from bulk water that we classically know. While this confinement effect is supposed to play a role in the fast diffusion of protons inside MXene materials, it has been impossible until now to characterize protons inside a MXene electrode during charging and discharging.
Vibrational modes analyzed
The team led by Dr. Tristan Petit at HZB has now succeeded in doing this for the first time by analyzing vibrational modes of protons excited by infrared light. Postdoctoral researcher Dr. Mailis Lounasvuori has developed an operando electrochemical cell that she used to analyze protons and water inside titanium carbide MXenes at BESSY II during the charging and discharging processes. In the process, she also succeeded in distilling out the special signature of the protons in the confined water between the MXene layers.
"These vibrational patterns are very different from those we would observe for protons in a three-dimensional water environment," says Mailis Lounasvuori.
"The fact that water molecules absorb infrared radiation particularly strongly while MXene emits very low amount of light in this energy range made IR spectroscopy ideally suited to our question," Petit explains.
Fast diffusion explained
This unusual hydration structure, showing that protons are solvated by fewer water molecules under confinement than in bulk water, suggest that proton de-solvation upon intercalation between MXene layers may contribute to pseudocapacitive energy storage in acidic environment.
It may also explain why protons diffuse particularly fast within MXene materials, which is related to their fast dis/charging time. Beyond energy storage applications, this work shows that MXenes are an ideal platform to investigate fundamental properties of confined chemical species, which certainly have other new chemical properties that remains to be discovered.
This technique will be further applied to other types of cations beyond protons (such as the Li+ ion) diffusing inside MXene materials to unravel new pseudocapacitive energy storage mechanisms.
More information: Mailis Lounasvuori et al, Vibrational signature of hydrated protons confined in MXene interlayers, Nature Communications (2023). DOI: 10.1038/s41467-023-36842-0. www.nature.com/articles/s41467-023-36842-0
Journal information: Nature Communications
Provided by Helmholtz Association of German Research Centres





















