A chemical reaction as good as gold for future technologies
A new Australian-led study finds gold atoms could be key to unlocking organic reactions.
Organic molecules are the building blocks for materials we use every day—from our clothes and coffee cups to the screen displays of our phones. Controlling reactions of these organic molecules is the key to designing materials with functional properties.
Reactions targeting carbon-hydrogen (C-H) bonds have long been of scientific interest given that almost all organic molecules contain these bonds. Led by FLEET at Monash University, a new study (published this week in the Journal of the American Chemical Society) finds that individual gold atoms may provide a low energy route for reactions which can target specific C-H bonds.
The 'holy grail' of chemical reactions
"One of FLEET's goals is the development of materials whose electronic properties may be exploited in low-energy technologies," says corresponding author A/Prof Agustin Schiffrin.
Organic molecules may serve as useful building blocks for tuneable construction of these materials, provided reactions between molecules can be controlled at the atomic scale.
Carbon-hydrogen bonds are among the most common bonds in organic molecules. Because of this, the ability to target specific C-H bonds in chemical reactions has been described by some researchers as the "holy grail." Unfortunately, two big challenges stand in the way of C-H activation reactions:
- Difficulty in targeting only one specific bond for reaction (poor selectivity).
- A lot of energy is required to break these bonds (high activation energy).
The Monash researchers have found that single gold atoms may provide a route to C-H activation.
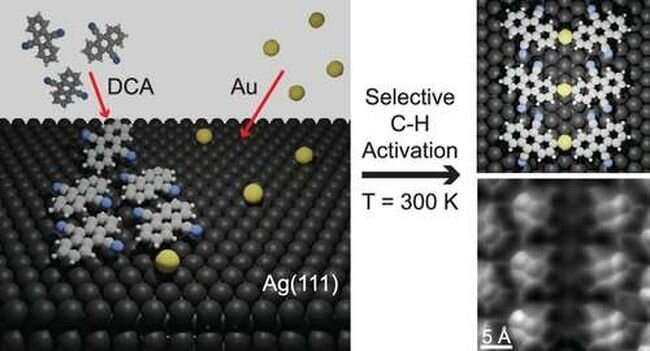
The researchers combined small numbers of individual gold atoms with organic 9,10-dicyanoanthracene (DCA) molecules on an atomically flat surface of silver, Ag(111).
"We used atomic-scale experimental techniques—scanning tunneling microscopy and atomic force microscopy—to image and characterize the samples," explains lead author Benjamin Lowe, who is a FLEET Ph.D. student at Monash. "These techniques revealed unusual covalent bonds between the carbon atoms of the DCA molecules and the gold atoms."
The formation of such covalent bonds suggested that specific C-H bonds had to have first been broken. Working with theoretical collaborators at the Czech Academy of Sciences, the researchers uncovered a reaction pathway which suggested that a metal-organic intermediate state formed by single gold atoms with pairs of DCA molecules could help such a reaction proceed.
Significantly, the reaction pathway uncovered can only explain C-H breaking of one specific C-H bond. The researchers found a dramatic decrease of the energy required to break this specific C-H bond (activation barrier), enabling the reaction at room temperature.
"This study directly addresses the two biggest challenges—namely poor selectivity and high activation barrier—that limit specific dissociation of C-H bonds in organic molecules," explains FLEET Chief Investigator Agustin Schiffrin. "Our approach can potentially open the door to the synthesis of novel organic and metal-organic nanomaterials, with properties useful for electronics, optoelectronics, sensing, catalysis, etc."
What's next?
Given the broad interest in reactions of organic molecules across a range of fields, this promising reaction has many potential applications such as polymer fabrication and modification of pharmaceutical products.
At FLEET, the researchers hope to exploit this selective and efficient reaction to produce atomically thin materials with desirable electronic properties.
The paper "Selective Activation of Aromatic C-H Bonds Catalyzed by Single Gold Atoms at Room Temperature" was published in the Journal of the American Chemical Society in November 2022.
The study was led by the School of Physics and Astronomy, Monash University, with co-authors from the Institute of Physics, Academy of Sciences of the Czech Republic, and Palacký University, Czech Republic.
More information: Benjamin Lowe et al, Selective Activation of Aromatic C–H Bonds Catalyzed by Single Gold Atoms at Room Temperature, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c10154
Journal information: Journal of the American Chemical Society
Provided by FLEET