New chemistry happens when dust meets pollution
It is a new chemistry found to take place in a cloud droplet, a wet aerosol, or on the surface of a dust particle. All that it takes to get started is natural events like dust storms, ocean wave action, volcanic eruptions, and wildfires, which increase the amount of aerosols in the atmosphere.
The effect of aerosols on climate may rival the warming of CO2 but depends on the chemical composition. Thus, measuring aerosols' size and "color" and how they change with time helps scientists in assessing their climate effect. These properties change because aerosols provide surfaces for water uptake and chemical reactions. Also, aerosols influence cloud formation and lifetime, and depending how high clouds are, they could cause heating or cooling.
Because of their diverse sources, atmospheric aerosols are chemically complex. They contain salts, organics, and transition metals. The latter originates from mineral dust, and iron is the most ubiquitous transition metal in these particles.
Plumes of mineral dust in the atmosphere are mixed with biomass burning smoke during long range transport following pollution events. Some of the organic carbon in biomass burning smoke is prone to oxidation and complexation with iron. However, the efficiency and nature of products from these reactions taking place under simulated aerosol and cloud conditions remain open research questions.
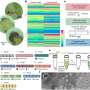
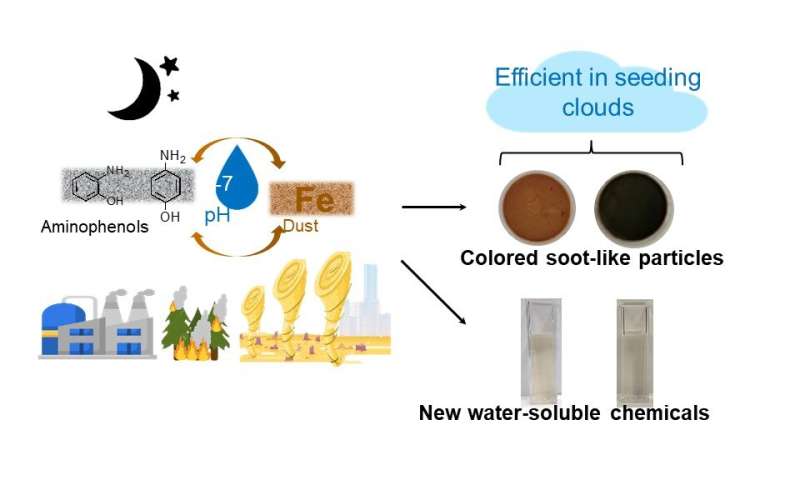
In a recent publication in Communications Chemistry, an international collaboration led by Hind Al-Abadleh from Wilfrid Laurier University, Marcelo Guzman from the University of Kentucky, and Akua Asa-Awuku from the University of Maryland focused on studying largely unexplored reactions of iron with aminophenols. The research carefully examined the role of aminophenols in forming colored nitrogen-containing organic carbon.
Aminophenols are examples of nitrogen-containing organic carbon, an important class of brown carbon whose contribution to climate forcing and aerosol-cloud interactions remains a large source of uncertainty in climate models due their chemical complexity and variable sources. These aromatic amines have been detected in the gas phase and ultrafine particulate matter from industrial emissions and from the reduction of nitrobenzenes and nitrophenols from biomass burning.
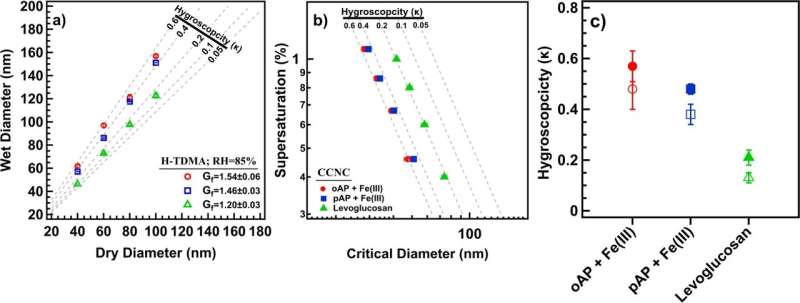
The new results in this publication show remarkably efficient formation of dark brown to black soot-like and water-soluble products under atmospherically-relevant conditions.
These products are oligomers containing 2-4 benzene rings with nitrogen and hydroxyl substituents from the abiotic iron-catalyzed oxidation of the aminophenols. The reactions were explored in homogeneous (i.e., aqueous phase) and heterogeneous systems (i.e, liquid/solid interface) using Arizona test dust. It was found that the hygroscopicity of the reaction products is higher than that of levoglucosan, a prominent proxy for biomass burning organic aerosol. The progressive darkening of Arizona test dust with reaction time was also reported, with clear changes to optical properties, morphology, mixing state, and chemical composition.
Metal-catalyzed chemistry is a poorly understood branch of atmospheric sciences despite the widespread presence of iron and other transition metals in particulate matter, in cloud and fog droplets, and on natural and engineered surfaces exposed to the air. The study highlights overlooked pathways that lead to the transformations of atmospheric aromatic amines in iron-containing dust systems.
These transformations affect cloud condensation nucleation efficiencies of multicomponent aerosol particles and change the physicochemical properties of the aerosols. These potentially important pathways are currently unaccounted for in climate and atmospheric chemistry models, and hence our results will help fill the gap in our understanding of the of the chemistry of iron in aerosols with various degrees of atmospheric processing.
More information: Hind A. Al-Abadleh et al, Reactivity of aminophenols in forming nitrogen-containing brown carbon from iron-catalyzed reactions, Communications Chemistry (2022). DOI: 10.1038/s42004-022-00732-1
Provided by University of Kentucky