Green hydrogen: Nanostructured nickel silicide shines as a catalyst
Electrolysis might be a familiar concept from chemistry lessons in school: Two electrodes are immersed in water and put under voltage. This voltage causes water molecules to break down into their components, and gas bubbles rise at the electrodes: Oxygen gas forms at the anode, while hydrogen bubbles form at the cathode. Electrolysis could produce hydrogen in a CO2-neutral way—as long as the required electricity is generated by fossil free energy forms such as sun or wind.
The only problem is that these reactions are not very efficient and extremely slow. To speed up the reactions, catalysts are used, based on precious and rare metals such as platinum, ruthenium or iridium. For large-scale use, however, such catalysts must consist of widely available and very cheap elements.
Chemically induced nanostructures
To accelerate the oxygen evolution reaction at the anode, nickel-based materials are considered as good candidates. Nickel is resistant to corrosion, hardly toxic and also inexpensive. Until now, however, energy-intensive high-temperature processes have been mostly used to produce nickel-based catalyst materials.
A team led by Dr. Prashanth Menezes (HZB/TU Berlin) has now found a "soft chemical" way to produce an efficient catalyst based on nickel-silicon intermetallic nanocrystals.
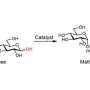
"We combined the element nickel with silicon, the second most abundant element in the Earth's crust, and achieved nanostructuring via a chemical reaction. The resulting material has excellent catalytic properties," says Menezes. The crystalline Ni2Si served as a precatalyst for the alkaline oxygen evolution reaction at the anode and undergoes surface transformation to form nicke(oxy)hydroxide as an active catalyst under operating condition. Remarkably, the water electrolysis was further paired with value added organic oxidation reaction in which electrosynthesis of industrially valuable nitrile compounds were produced from primary amines with selective and full conversion under mild conditions. Such electrosynthetic methods can boost up the hydrogen generation at the cathode and can simultaneously provide access to valuable industrial products at the anode.
More efficient and stable
Compared to modern catalysts based on Nickel, Cobalt, Iron, Ruthenium and Iridium, the nanoporous Ni2Si is significantly more active and remains stable for longer reaction time at industrial-level conditions. To understand the behavior of Ni2Si in more detail, the team combined different measurement methods, including elemental analyses, electron microscopy and modern spectroscopic measurements at BESSY II. "In the future, even industrial alkaline water electrolyzers could be equipped with a coating of this nanoporous nickel silicide," says Menezes.
The research was published in Advanced Energy Materials.
More information: Indranil Mondal et al, Nanostructured Intermetallic Nickel Silicide (Pre)Catalyst for Anodic Oxygen Evolution Reaction and Selective Dehydrogenation of Primary Amines, Advanced Energy Materials (2022). DOI: 10.1002/aenm.202200269
Journal information: Advanced Energy Materials
Provided by Helmholtz Association of German Research Centres