Team develops quality control for single cell imaging
One size doesn't necessarily fit all when it comes to testing the effects, including cancer and other diseases, of endocrine-disrupting chemicals (EDC) or toxins, which target many factors, including the estrogen receptor (ER). Most current screening uses high throughput assays that only focus on bulk population analysis and/or engineered systems without taking into account phenotypic heterogeneity or cell-to cell-variability of endogenous receptors. Researchers at Baylor College of Medicine have now developed a two-tiered image analysis-based quality control pipeline that exploits single cell variations in evaluating chemical effects.
The findings, published in a recent edition of Environmental Health Perspectives, found that when using this method, certain EDCs were only active on the endogenous ER, which were not detected by current assays.
"The majority of tests currently used to identify the effects of EDCs on ERs do not consider individual cell responses within the cell population, and although this is an important biological phenomenon, very few studies have attempted such experiments," said Dr. Michael Mancini, senior author on the paper, professor of molecular and cellular biology and director of the Integrated Microscopy Core at Baylor. "When it comes to screening tools for most toxicology assays, many people want a single answer or number from thousands and sometimes millions of cell responses. However, the individual cell responses are not all identical, so we focused on an assay model where each individual cell is analyzed."
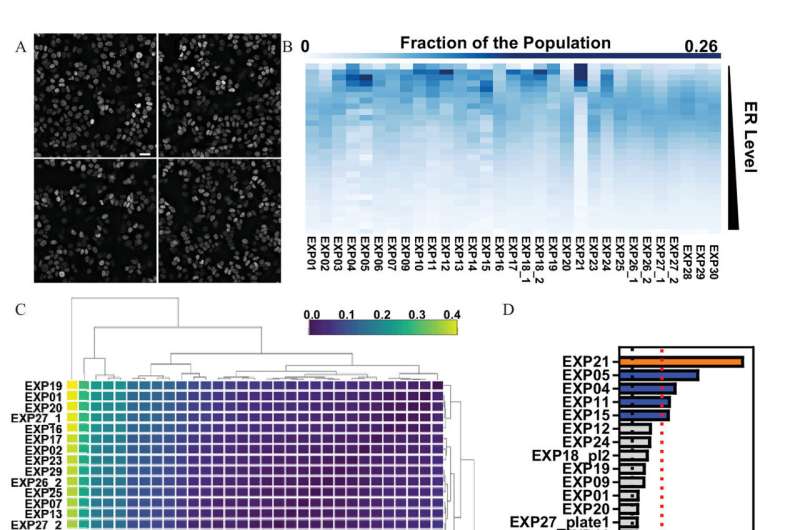
Dr. Fabio Stossi, associate professor of molecular and cellular biology and lead author of the study explains, "In this study we measured endogenous ER levels in thousands of cells to create a quality control method to evaluate the reproducibility of cell-to-cell variation across multiple tests. We measured the cell-by-cell variability using imaging techniques to determine reproducibility across many factors and variables, and then we found ways to measure the changes of distribution when you treat the cells with EDCs.
Over the course of several years, we have used this method to create a pipeline and software for others to use in quality control of single cell imaging experiments."
To determine the accuracy of this method, Stossi and his collaborators compared results to several assays currently used by the Environmental Protection Agency and by using 42 toxicants from the Agency for Toxic Substance and Disease Registry. These EDCs are common in the environment and are known to be linked to certain types of cancers and other illnesses.
During testing, researchers found that using this new method for single-cell imaging analytics, coupled with current assays, they were able to identify novel EDCs that specifically affected endogenous ER levels, an effect that involves interrelations between multiple pathways.
"We identified several new toxicants that directly and indirectly affected ER levels and activity," Mancini said. "We were able to see massive heterogeneity and diversity among similar cell populations that in the past were believed to act in similar fashion. They don't, so when most people are conducting a set number of experiments looking at only certain outputs, they aren't necessarily getting the most accurate results. What our team did was to create this imaging assay able to find the most accurate results by employing single-cell analytics across a population of cells."
Since this platform of testing can be integrated with most assays the government currently uses to test toxicants, the next step will be to examine other EDC-targeted receptors in different models and to utilize new equipment that supports larger-scale studies.
Others who contributed to the study include Pankaj K. Singh, Ragini M. Mistry, Hannah L. Johnson, Radhika D. Dandekar, Maureen G. Mancini, Adam T. Szafran and Arvind U. Rao, with Baylor College of Medicine, Texas A&M University or the University of Michigan, Ann Arbor.
More information: Fabio Stossi et al, Quality Control for Single Cell Imaging Analytics Using Endocrine Disruptor-Induced Changes in Estrogen Receptor Expression, Environmental Health Perspectives (2022). DOI: 10.1289/EHP9297
Journal information: Environmental Health Perspectives
Provided by Baylor College of Medicine