Enzyme ST6Gal-I modulates cell mechanics and enhances invasion by cancer cells
For more than two decades, University of Alabama at Birmingham researcher Susan Bellis, Ph.D., has studied how the addition of sialic acid to various proteins increases cancer resistance and oncogenicity.
One of the enzymes that transfers sialic acid to target glycoproteins is ST6Gal-I, and it has attracted increased attention in the cancer field in recent years. ST6Gal-I is upregulated in breast cancer, gliomas, pancreatic cancer, prostate cancer and ovarian cancer, and it plays a key role in tumor progression and metastasis.
Metastasis is the spread of a tumor to other parts of the body, through the migration of tumor cells. Cells move themselves through cell adhesion mechanics—integrins at the cell membrane can attach themselves to a surface, acting as tiny anchors. The cell's cytoskeleton then pushes the front of the cell forward to establish new anchors, and the now-rear anchors let go. Such cell mobility is vital in embryogenesis, development and wound healing. However, in cancer, cell migration can be deadly.
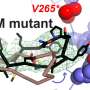
UAB researcher Alexa Mattheyses, Ph.D., is able to study cell adhesion mechanics directly, using DNA tension-gauge tether probes displaying an integrin ligand and attached to a coverslip surface. When a cell binds to the tension probe and exerts force, the DNA duplex separates, generating a fluorescent signal whose changes are monitored by sophisticated fluorescence microscopy. Two years ago, the Mattheyses lab showed that activation of the epidermal growth factor receptor, or EGFR, by its ligand—epidermal growth factor, or EGF—modulated integrin forces and attenuated the mechanical threshold for integrin tension and formation of focal adhesions. Focal adhesions are the mechanical linkages, the anchors, to the extracellular matrix outside the cell. They are also the place where mechanical force and regulatory signals are transmitted. A cell-surface receptor like EGFR transfers a signal from its external ligand to the interior of the cell.
The Mattheyses and Bellis labs collaborated to expand their EGFR work by looking at the effect of adding sialic acid to EGFR on cell mechanics. In a study published in the Journal of Biological Chemistry that included tests of three types of human cancer cells, they report that ST6Gal-I–mediated sialylation of the EGFR modulates cell mechanics and enhances invasion by the cancer cells.
"Given the widespread impact of sialylation and the prognostic value of ST6Gal-I expression, an improved understanding of how ST6Gal-I–mediated sialylation alters cell mechanics may open the door to a new range of cancer therapeutics," Mattheyses said. "Our results help bridge the mechanistic gap in the field, while demonstrating the potential value in oncogenic mechanosignaling as a therapeutic target."
"Clinically, increased glycoprotein sialylation has been associated with carcinogenesis, and ST6Gal-I promotes vital cancer hallmarks such as self-renewal, invasiveness, proliferative potential and resistance to cell death," Mattheyses said. "While mechanical changes in cells and tissues also contribute to malignancy and metastasis, the underlying mechanisms by which these changes promote cancer have remained understudied."
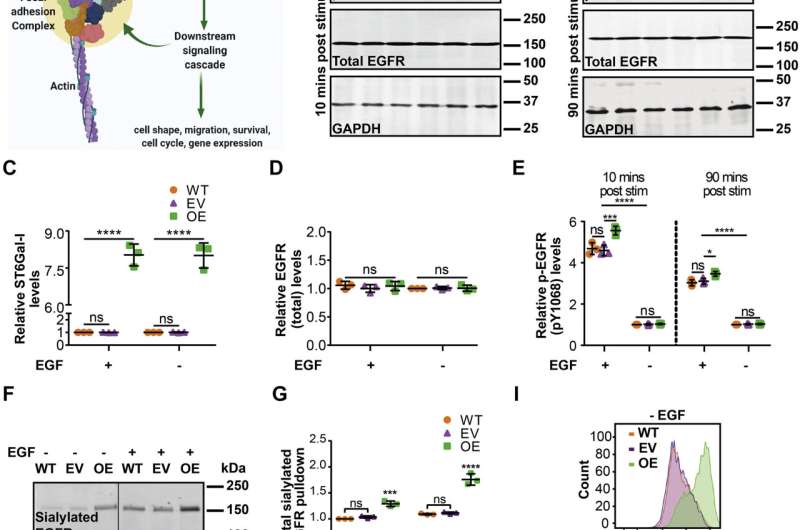
The UAB researchers used primate kidney cells as a test-bed system, and three types of human cancer cells. In cells with little or no ST6Gal-I, they introduced and overexpressed the enzyme. In cells that expressed ST6Gal-I, they knocked down expression. They then compared overexpressing and poorly expressing cells, using DNA tension-gauge tethers and fluorescence microscopy.
They found that ST6Gal-I overexpression promoted cell spreading and focal adhesion maturation in an activated EGFR-dependent manner. The cells' force histories, as reported by the DNA tethers, showed that ST6Gal-I overexpression led to increased tension generation by integrins. Classical cancer biology assays showed that ST6Gal-I overexpression enhanced mechanosignaling-increased migration, invasion, proliferation and survival.
The researchers also examined the downstream EGFR-signaling cascades that might regulate mechanical outcomes or alterations in cell morphometrics, which is the quantitative analysis of form. They found that changes in cell mechanical properties—such as integrin tension, focal adhesion nucleation and promotion of cell spread area—depended on the extracellular-signal-regulated kinase, or ERK, pathway. In contrast, increases in cellular migration, invasion, proliferation and survival were controlled via the phosphoinositide 3-kinase-Akt serine/threonine kinase, or AKT, cascade.
They also found that high ST6Gal-I activity led to sustained EGFR membrane retention, making it a key regulator of cell mechanics.
"Our findings suggest a novel sialylation-dependent mechanism orchestrating cellular mechanics and enhancing cell motility via EGFR signaling," Mattheyses said.
More information: Tejeshwar C. Rao et al, ST6Gal-I–mediated sialylation of the epidermal growth factor receptor modulates cell mechanics and enhances invasion, Journal of Biological Chemistry (2022). DOI: 10.1016/j.jbc.2022.101726
Journal information: Journal of Biological Chemistry
Provided by University of Alabama at Birmingham