This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Biophysicists decipher functionality of adrenaline-binding receptor
G protein-coupled receptors (GPCRs) are found throughout the human body and are involved in many complex signaling pathways. Despite their importance in many biological processes, the central mechanism of G protein-coupling and the associated signal transmission is not yet understood.
A team of researchers from Leipzig University has succeeded in understanding the mechanism of signal transmission through an adrenaline-binding receptor at the atomic level. In the future, researchers may be able to use these results to better avoid side effects when developing drugs.
The study has been published in the journal Nature Structural & Molecular Biology.
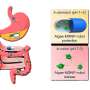
Every organism reacts to its environment. An external stimulus causes the body to release messengers such as adrenaline, which bind to receptors. The receptors transmit the signal to other proteins. This triggers biochemical cascades that lead to a response in the organism, such as a flight-or-fight response in case of the adrenaline-binding receptor.
Drugs are often modeled based on these messengers and work by interacting with receptors. Side effects can occur if the drug binds to the wrong receptor or does not transmit the signal to the correct intracellular protein. To prevent this, scientists are studying how receptors work.
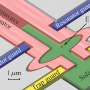
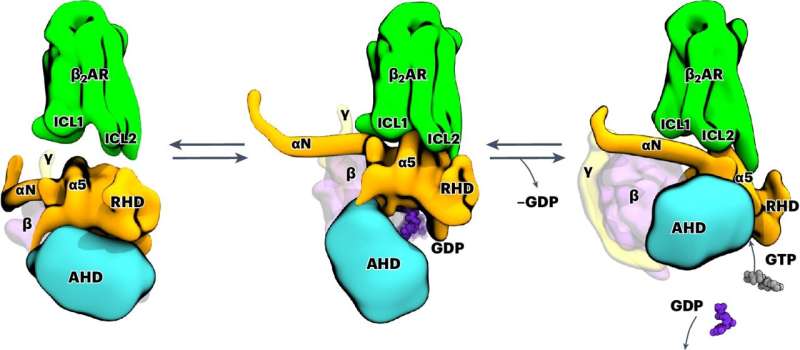
In the current study, Professor Peter Hildebrand and his team from the Institute of Medical Physics and Biophysics at Leipzig University show how signal transmission through the β2 adrenergic receptor takes place at the atomic level. This is a G protein-coupled receptor (GPCR). The members of this protein superfamily are embedded in the cell membrane.
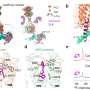
The team used computer-aided molecular dynamics simulations as well as biochemical and functional mutation analyses for their investigations. This allowed them to observe how the receptor works: by binding, the receptor changes the three-dimensional structure of intracellular G protein, which then releases the regulatory molecule GDP.
In the next step, this G protein can be activated by binding its actual substrate GTP and trigger biochemical cascades in the cell. The team of researchers also found that the exact function of the receptor depends on the arrangement of various flexible structural elements. They cannot be characterized using classical structural biology methods.
Professor Hildebrand is now planning to apply the computer-aided biophysical methods to other receptor systems, such as in obesity research, a focus of medical research at Leipzig University. "Comparative studies of dynamic signaling are exciting when drugs with different profiles are used," explains the professor of biophysical computer simulations.
Professor Peter W. Hildebrand has been researching receptors at the Faculty of Medicine at Leipzig University since 2017. From 2008–2014, he studied the structure of the photoreceptor rhodopsin with Professor Klaus-Peter Hofmann and Dr. Patrick Scheerer at the Charité.
He is now also collaborating with Nobel laureate Professor Brian Kobilka and cryo-electron microscopist Professor Yiorgo Skiniotis, Stanford University, U.S., to better understand GPCR-mediated signaling. Together, they recently elucidated the mechanism of GTP binding to the G protein and its activation, and published the results in Nature.
"For the first time, we now have a comprehensive picture of the structural mechanism of receptor-mediated signaling from the outside to the inside of the cell," says Hildebrand. "Alongside my collaborators, I owe this success above all to the talented young scientists Dr. Hossein Batebi and Dr. Guillermo Pérez-Hernández from my team."
More information: Hossein Batebi et al, Mechanistic insights into G-protein coupling with an agonist-bound G-protein-coupled receptor, Nature Structural & Molecular Biology (2024). DOI: 10.1038/s41594-024-01334-2
Journal information: Nature Structural & Molecular Biology , Nature
Provided by Leipzig University