This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers 'film' the activation of an important receptor
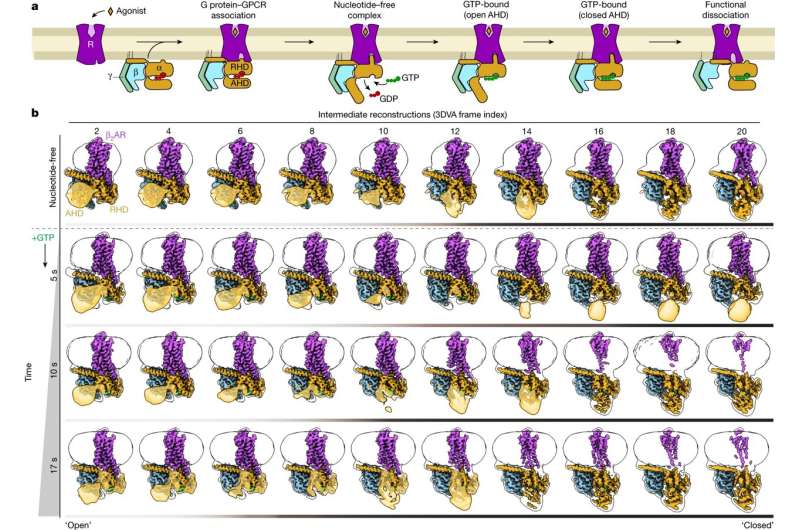
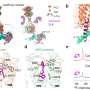
An international team of researchers has succeeded in "filming" the activation of an important receptor. They froze the involved molecules at different points in time and photographed them under the electron microscope. They were then able to place these still images in sequence. This sequence shows step by step which spatial changes the receptor undergoes when it is activated.
Researchers at Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) contributed substantially to the study. The results, which have now been published in the journal Nature, may lead in the medium term to the development of more effective medicines.
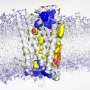
Cells communicate with each other via signal molecules that they detect using specific receiving structures known as receptors. These are embedded in the cell membrane, the thin layer that surrounds the cell. One particularly important group of receptors are known as GPCRs.
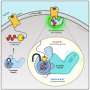
If a suitable signal molecule attaches to their exterior, a complex chain of reactions is set in motion. The receptor changes its spatial structure, thereby activating a G-protein on the inside of the cell that is attached to the receptor. This protein moves away and can then, for example shuttles to an enzyme in the cell by diffusion to regulate this enzyme or it may switch the transcription of certain genes on or off.
"Humans have more than 800 GPCRs, each of which is specialized for detecting a particular signal," explains Prof. Dr. Peter Gmeiner, Chair of Pharmaceutical Chemistry at FAU. "In our study we focused on one particular GPCR– the β2-adrenergic receptor. It is activated by adrenaline and is involved, for example, in the regulation of heart and lung function."
It is therefore also an important possible starting point for developing medicine for treating asthma or cardiac insufficiency. "In order to do so, however, it is important to have a comprehensive understanding of the activation of the receptor and the G-protein attached to it," explains Gmeiner.
The results that have now been published may make a significant contribution. The international team led by Georgios Skiniotis (Stanford University) and including Brian Kobilka (Stanford University), Peter Hildebrand (Universität Leipzig and Charité Berlin) and Peter Gmeiner successfully broke down the process involved in the receptor activation, step by step. The researchers used a special method known as time-resolved cryogenic electron microscopy. The complex consisting of the receptor and G-protein is shock-frozen at -150 degrees shortly after activation.
"Under the microscope, we obtain a series of different frames," Gmeiner explains. "Different, because the thousands of molecules we observe under the microscope are never entirely synchronous. Their natural mobility means that some are frozen at a slightly earlier stage of activation and others at a more advanced stage."
This "shock-freezing" can be repeated at different times after activation. The images gained as a result allow researchers to reconstruct the process step by step, at the atomic level.
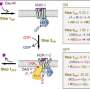
"In our work, we focus predominantly on changes to the spatial structure of the G-protein triggered after the drug attaches to the β2-adrenergic receptor," explains Gmeiner. His group made a major contribution to the success of the project: They succeeded in creating a type of "super-adrenaline" that binds particularly well to the β2-receptor.
"This strong bond stabilizes the complex consisting of the receptor and G-protein," explains the scientist from FAU. Normally, this task is the responsibility of proteins known as adapter proteins. They act like molecular chewing gum and hold the complex together.
"However, they do their job so well that no interim steps are visible under the cryogenic electron microscope," explains Gmeiner. Thanks to his "super adrenaline," the researchers were able to do without adapter proteins. The receptor-G protein complex is stable enough without them.
"Only then were we able to make the movement visible," Gmeiner adds.
The results may facilitate the development of new medicines, and not only those that have an effect on the β2 adrenergic receptors. GPCRs are considered to play a central role in fighting disease. Almost one-third of today's approved drugs influence the function of these receptors, for example by strengthening or weakening the transmission of signals to cells. Time-resolved cryogenic electron microscopy should make it easier to develop particularly effective drugs tailored to a specific need and therefore with fewer side effects, Gmeiner, hopes.
It is vital that researchers completely understand the molecular processes of the receptors and their G-proteins for this to be the case. The Nobel Prize won several years ago by Brian Kobilka, one of the researchers involved in the current program, shows how important this is. He was the first to determine the three-dimensional structure of a GPCR using X-ray crystallography, at an atomic scale and in three different states. A tailor-made drug developed by Peter Gmeiner's laboratory was also used in these experiments.
More information: Makaía M. Papasergi-Scott et al, Time-resolved cryo-EM of G-protein activation by a GPCR, Nature (2024). DOI: 10.1038/s41586-024-07153-1
Journal information: Nature
Provided by Friedrich–Alexander University Erlangen–Nurnberg