Molecular powerhouse of the cell division motor
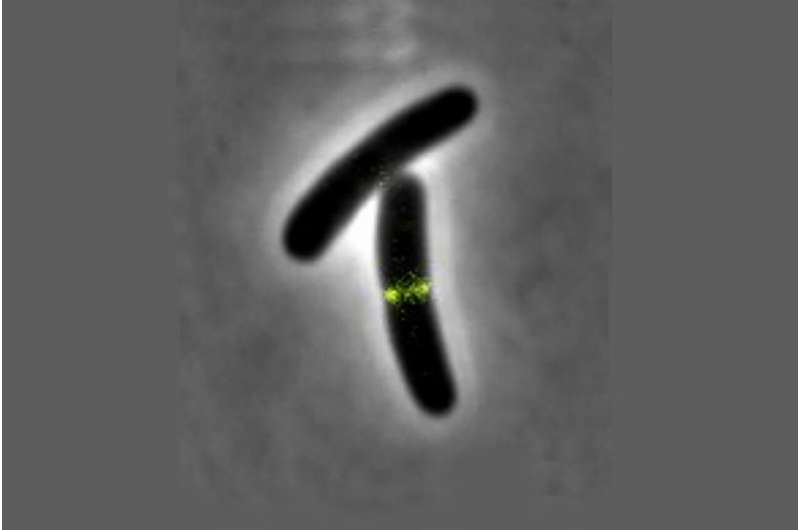
All living cells must grow and divide in order to multiply. The multiplication of bacteria normally occurs through so-called binary fission. This makes particularly rapid growth possible and is the reason why bacteria, including microbial pathogens, can multiply exponentially.
The molecular mechanisms involved in cell division are very complex and not yet fully understood. An important current question in this research field is how the force necessary to deform the cell membrane and thus for the division of a cell is exerted and how it acts on the cell envelope. At Kiel University, the Microbial Biochemistry and Cell Biology research group led by Professor Marc Bramkamp is working on bacterial organizational and reproductive mechanisms, among other things. The research team from the Institute of General Microbiology at Kiel University worked closely with Professor Petra Schwille's group at the Max-Planck-Institute of Biochemistry in Martinsried.
Using the bacterium Escherichia coli as an example, both groups have now succeeded in gaining new insights into the role played by the cell division molecule FtsZ in initiating this process. The interdisciplinary research team from Martinsried and Kiel was able to show that FtsZ can generate the force for membrane deformation by means of a process known as "treadmilling" and thus initiate the process of cell division. The new results on the role of the protein as a molecular initiator of cell division were published jointly by the researchers today in the renowned journal Nature Communications.
Torsional force deforms cell membrane
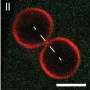
Before a bacterial cell can divide, the multi-layered cell envelope consisting of cell membrane and cell wall must be doubled at the division site. In addition, the cell is under an osmotic internal pressure, the so-called cell turgor. To prevent the cell from bursting during division, the stability of the cell envelope must therefore be guaranteed at all times. In order to investigate the question of force transmission for the deformation of the cell membrane in this complex process, Bramkamp's working group collaborated with Schwille's team from Martinsried. The starting point for the investigation were results obtained by the Max-Planck-researchers using a model system: They investigated the effect of a modified FtsZ protein on an artificial cell membrane in a test tube and were able to prove in principle that the membrane deformation caused by the protein actually occurs as a physical force and can be measured.
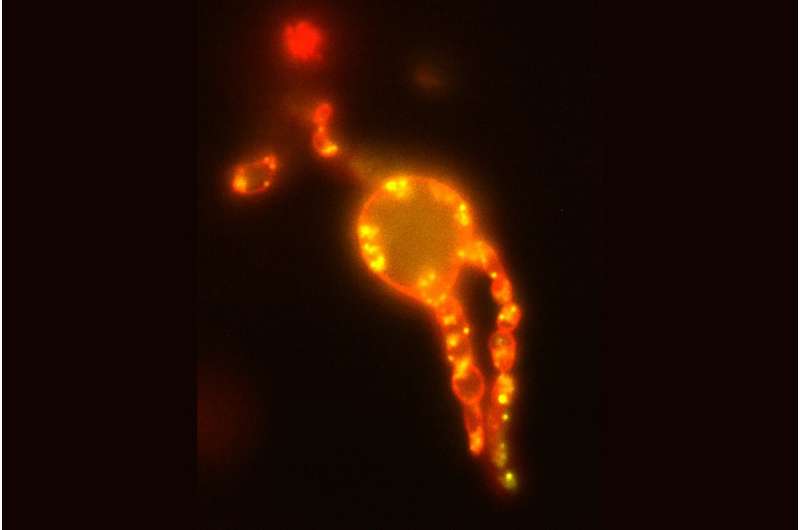
This is where the scientists from Kiel Microbiology came in to test these basic principles for their validity in a living cell. "In order to be able to precisely observe possible effects on the cell membrane in a living organism, we first used a trick and removed the cell wall of E. coli cells and transferred them into an isotonic medium in which they can survive without internal pressure but otherwise normally," explains co-author Fabian Meyer, a doctoral student in Bramkamp's research group. "Subsequently, we were able to confirm that anchoring of the FtsZ protein to the membrane of an E. coli cell actually takes place in vivo as well. The prerequisite for force transmission by the protein was thus fulfilled. We were then able to observe its effects even better in the absence of the counterpressure emanating from the cell turgor."
In a next step, the Kiel researchers investigated how the "treadmilling" mechanism, whose structure has been known for some years, has a functional effect on the bacterial cell membrane. The FtsZ protein forms a ring-shaped structure to which other enzymes required for cell division can attach. In the process, building blocks are continuously incorporated into the filament structure, while others are released again at the opposite end of the filament, so that the entire structure virtually rotates around itself in a circle.
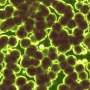
"The question was now whether the FtsZ can also generate torque in vivo via this 'treadmillling' that is suitable for deforming the cell membrane," Meyer says. "With the help of three-dimensional fluorescence microscopy, we were able to confirm that the force exerted by the FtsZ actually takes place in the presence of all the other proteins of a living cell in exactly the same way as in the model. We were thus able to directly observe how the protein pinches off individual membrane components and thus initiates the directional change of the membrane that is necessary for division." The research teams around Prof. Schwille and Prof. Bramkamp thus succeeded in proving that the dynamics of the FtsZ protein indeed exert a force on the membrane in the living organism as well—and that this force is sufficient to bend the membrane.
Studying cellular self-organization using imaging
The new results of the research cooperation between Kiel University and the Max-Planck-Institute of Biochemistry demonstrate once again the great value of interdisciplinary collaborations: only the joint efforts of biophysical and molecular biological experts made it possible in this case to decipher another building block of the molecular architecture of life in the form of the analysis of the FtsZ protein and its role in membrane deformation. From Kiel's point of view, the application of microscopic analyzes again played a major role: "Our imaging methods, in this case three-dimensional fluorescence microscopy, allow us here and in other cases to gain insights into life processes that go far beyond biochemical analyzes," says Bramkamp, head of the Microbial Biochemistry and Cell Biology research group at Kiel University. "Biochemically, the binding of the FtsZ protein to an energy carrier results in the release of energy. Only the observation of the protein in the living cell allows us to understand the effect of this energy release—which in this case manifests itself in the deformation of the cell membrane."
Overall, the new research contributes to the efforts of the Microbial Biochemistry and Cell Biology group to identify generally valid principles of biological pattern formation. This pattern formation is of central importance in biology, for example in the development of complex multicellular organisms.
More information: Diego A. Ramirez-Diaz et al, FtsZ induces membrane deformations via torsional stress upon GTP hydrolysis, Nature Communications (2021). DOI: 10.1038/s41467-021-23387-3
Journal information: Nature Communications
Provided by Kiel University