How cells recycle the machinery that drives their motility?
Research groups at University of Helsinki and Institut Jacques Monod, Paris, discovered a new molecular mechanism that promotes cell migration. The discovery sheds light on the mechanisms that drive uncontrolled movement of cancer cells, and also revises the 'text book view' of cell migration.
The ability of cells to move within our bodies is critical in wound healing, as well as for immune cells to patrol in our tissues to hunt bacterial and viral pathogens. On the flip-side, uncontrolled movement of cells is a hallmark of cancer invasion and metastasis.
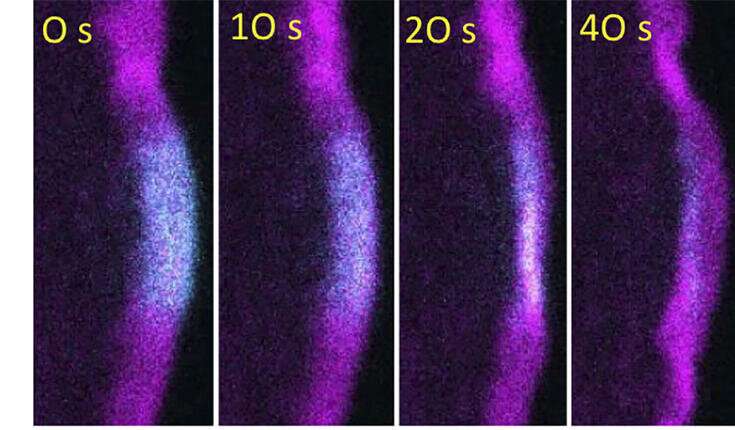
The machinery that drives cell migration is a complex network of dynamic filaments composed of a protein actin. Actin exists in monomeric form, but like Lego bricks, different types of filamentous structures can be built from actin monomers in cells. Actin filaments are organized in cells in a way that their rapidly elongating plus-ends face the plasma membrane, whereas their minus-ends are oriented away from the plasma membrane. Elongation of actin filaments at their plus-ends against the plasma membrane generates the force to push the leading edge of cell forward during cell migration. To maintain a sufficient supply of monomeric actin subunits for filament elongation, actin filaments must be rapidly disassembled in cells, and this is believed to occur at their minus-ends. An important factor that limits actin filament disassembly to their minus-ends is Capping Protein, which binds very tightly to filament plus-ends to block filament elongation and shortening (see related figure).
A new study published in Nature Cell Biology reveals that this 'text book view' of cell migration needs to be revised. The research, led by Academy Professor Pekka Lappalainen from HiLIFE Institute of Biotechnology, University of Helsinki, revealed that a conserved actin-binding protein, Twinfilin, efficiently removes Capping Protein from the filament plus-ends ends. This leads to filament depolymerization also from their 'aged' plus-ends, which no longer push the leading edge of cell forward. In the absence of Twinfilin, actin filament recycling is diminished, filaments push the cell edge forward less efficiently, and cell migration is slower.
"Our results suggest that Twinfilin and Capping Protein make together a 'molecular clock', which ensures that the 'productive' actin filaments pushing the plasma membrane have a sufficient supply of actin monomers, whereas the 'aged' actin filaments that no longer push the plasma membrane are disassembled," says Lappalainen.
"This study highlights the need of several proteins with different functions to act in synergistic manner to maintain the normal morphology and functions of actin networks in cells," continues Dr. Markku Hakala who is the main author of this study.
Despite extensive studies, the precise mechanisms by which actin monomers are recycled in cells has remained elusive. The new study adds an important piece in this puzzle by reveling how Capping Protein is removed from actin filament plus-ends to enable their rapid disassembly. These findings also create a basis for further studies to understand how irregularities in actin disassembly machinery cause severe diseases and developmental disorders.
"Uncontrolled expression of Twinfilin is linked to many diseases, such as breast cancer invasion and lymphoma progression. Our work, therefore, also sheds light on the molecular mechanisms that drive uncontrolled movement of cancer cells," concludes Lappalainen.
More information: Markku Hakala et al, Twinfilin uncaps filament barbed ends to promote turnover of lamellipodial actin networks, Nature Cell Biology (2021). DOI: 10.1038/s41556-020-00629-y
Journal information: Nature Cell Biology
Provided by University of Helsinki