Researchers develop high-performance ceramic fuel cell that operates on butane gas
A Korean research team has developed a high-performance ceramic fuel cell that can operate on butane fuels. Since butane can be liquefied and thus stored and transported easily, the new technology could expand the application range of ceramic fuel cells to portable and mobile applications such as electric cars, robots and drones. Previously, ceramic fuel cells had only been considered for application to large-capacity power generation systems due to their high-temperature operation.
The Korea Institute of Science and Technology (KIST) announced that Dr. Son Ji-Won's research team at KIST's Center for Energy Materials Research had developed a high-performance, thin-film-based ceramic fuel cell that could operate at mid-to-low temperatures below 600 °C using butane fuels.
Ceramic fuel cells are a type of high-temperature fuel cell that operates over 800 degrees C. This high temperature allows the use of inexpensive catalysts, such as nickel, in contrast to low-temperature fuel cells, such as polymer electrolyte fuel cells, which use high-priced platinum catalysts to supplement their low catalytic activity. Another major advantage of high-temperature fuel cells is that they can various fuels other than pure hydrogen, such as LPG and LNG with low emission due to high efficiency. However, ironically, even though high-temperature fuel cells use inexpensive catalysts, their operation requires expensive refractory materials and manufacturing technologies. Another limiting factor is that their system on-off process takes a long time due to the characteristics of high-temperature operation, which restrict their application to large-scale stationary power generation systems.
Many research teams around the world have worked on thin-film-based ceramic fuel cells, which can operate at low temperatures without performance loss. Unfortunately, the problem is that lower-temperature operation causes ceramic fuel cells to lose one of their important advantages, that is, their ability to use various fuels. When the nickel catalyst of ceramic fuel cells is used with hydrocarbon fuels, such as methane, propane, and butane, the carbon generated during fuel conversion is deposited on the surface of nickel. This worsens seriously as the temperature lowers, leading to the failure of the cell operation.
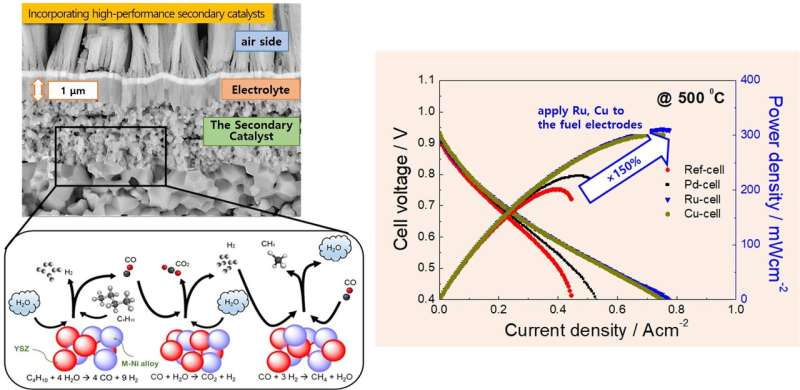
Dr. Son Ji-Won's research team solved this problem by incorporating high-performance secondary catalysts, which can convert fuels more easily, by thin-film technology. Using alternating deposition of the secondary catalyst and the main catalyst layers, the team was able to effectively distribute the secondary catalyst at the nearliest parts of the fuel electrodes to the electrolyte. By this way, controlled incorporation of small amount but effectively positioned secondary catalysts was possible.
Using this procedure, the KIST research team was able to successfully apply secondary catalysts known for their high catalytic activity at low temperatures, such as palladium (Pd), ruthenium (Ru), and copper (Cu), to the nanostructure fuel electrodes. They confirmed the high-performance operation of the newly developed thin-film-based ceramic fuel cells at mid and low operation temperatures (500-600 C), using butane fuel, which is a very affordable fuel.
"This research systematically examined the possible uses of hydrocarbon fuels in ceramic fuel cells operating at low temperatures," said Dr. Son Ji-won. "The use of the portable fuels like butane at lower operating temperatures would enable the development of smaller and integrated ceramic fuel cell systems, which can be applied to portable and mobile power sources."
More information: Cam-Anh Thieu et al, Effect of secondary metal catalysts on butane internal steam reforming operation of thin-film solid oxide fuel cells at 500–600 °C, Applied Catalysis B: Environmental (2019). DOI: 10.1016/j.apcatb.2019.118349
Journal information: Applied Catalysis B: Environmental
Provided by National Research Council of Science & Technology