Nuclear pore complex outer rings: No longer 'one size fits all'
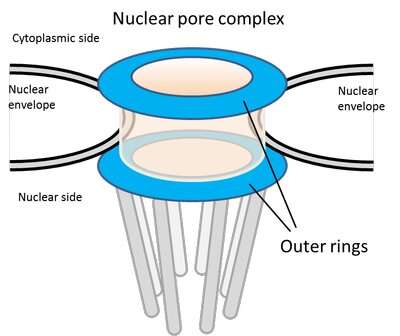
In eukaryotic cells, the nucleus is walled off from the rest of the cell by the nuclear envelope. All transport into and out of the nucleus occurs via cylindrical channels called nuclear pore complexes (NPCs) that penetrate the nuclear envelope. Each NPC is made up of eight repeating protein complexes containing at least 30 different types of proteins, called nucleoporins (Nups). These complexes fit together like the wedges of an orange, leaving a channel in the middle through which proteins, RNA, and signaling molecules can be transported.
The Nups at either end of the NPC form ring-like structures that frame the openings of the channel. So far, all work in eukaryotic cells has suggested that these outer rings are identical, comprising equal numbers of nine or ten different Nups linked together in repeating Y-shaped structures.
But in a study published this week in PLoS Genetics, a team lead by researchers from Osaka University and National Institute of Information and Communications Technology (NICT) found that the "one size fits all" theory may not hold true.
"We wanted to take a closer look at the NPCs of fission yeast, Schizosaccharomyces pombe", says lead author of the study Haruhiko Asakawa at Osaka University. "Like other eukaryotes, S. pombe has a conserved set of Nups, but unlike these other species, the outer ring structures are made up of unequal numbers of the different types of proteins."
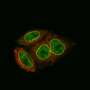
And while the researchers were expecting some differences, they weren't quite prepared for the completely divergent structures revealed by using high-powered immunoelectron and fluorescence microscopes. By carefully tracking the locations of the different proteins, they made their startling discovery.
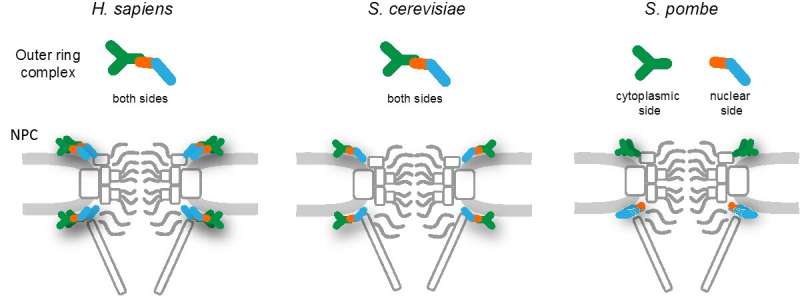
"It's like S. pombe has completely thrown the rule book out the window," laughs senior author Tokuko Haraguchi at NICT. "We found that the proteins that make up the outer ring structures were arranged asymmetrically. Rather than having identical structures, the nuclear outer ring comprised only two types of Nups, with the seven remaining Nups all part of the cytoplasmic outer ring structure."
Interestingly, the researchers found that the asymmetrical ring structure was essential for normal cell growth in S. pombe.
"This diversity in the outer ring structures may provide clues as to how the nuclear pore is formed, along with insights into the structure and function of the cell nucleus from an evolutionary point of view," says Asakawa. "We also hope that our findings will help us to better understand the mechanisms of diseases caused by abnormal nuclear pore proteins, which in turn may lead to new treatment strategies."
More information: Haruhiko Asakawa et al, Asymmetrical localization of Nup107-160 subcomplex components within the nuclear pore complex in fission yeast, PLOS Genetics (2019). DOI: 10.1371/journal.pgen.1008061
Journal information: PLoS Genetics
Provided by Osaka University