How cytoplasm separates from yolk
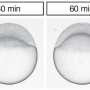
The segregation of yolk from the surrounding cytoplasm in the very early fish embryo is a key process for the development of fish larva. To identify its underlying mechanisms, biologists at the Institute of Science and Technology Austria (IST Austria) teamed up with their colleagues in theoretical physics. The discovery: Actin dynamics in the bulk of the cell drive phase segregation in zebrafish oocytes.
A single-cell fish egg evolves into a multi-cell embryo in less than two hours after fertilization. Within these two hours, the cytoplasm, which will later form the animal body, must separate completely from the yolk, which the larva is going to feed on. Previously, cell biologists had proposed that local expansion of the cell surface at one pole of the egg mediates this segregation. However, direct evidence supporting this model was lacking.
Joined forces: lab experiments and physical theory
To understand the physical basis of this segregation process, Shayan Shamipour, Ph.D. student in the research group of developmental biologist Carl-Philipp Heisenberg, teamed up with the research group of theoretical physicist Edouard Hannezo. Based on the combined expertise of these two groups, the authors, also including a third professor from IST Austria, Björn Hof, reveal that the forces exerted at the cell surface are not essential for this process—as opposed to previous models. Instead, they discovered that combined pulling and pushing forces within the embryo facilitate the segregation of cytoplasm from the yolk granules. Importantly, the theory developed to describe this process can be applied to any segregation due to the forces exerted from an active fluid and could thus also be used to examine similar processes in mammalian/human embryos.
Size matters
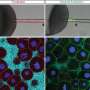
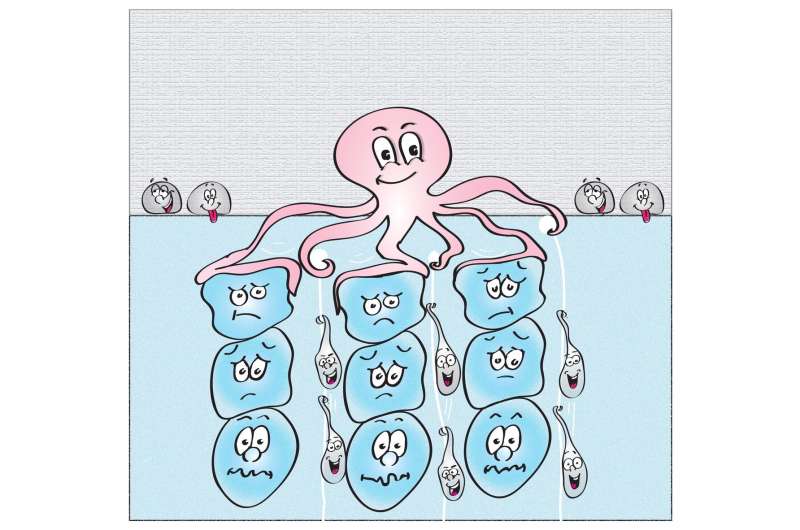
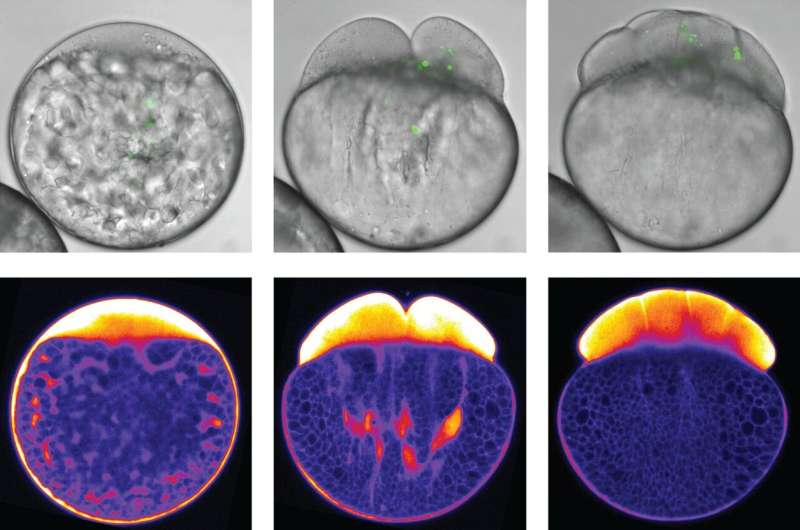
But how are these concerted pulling and pushing movements generated? In the bulk of the cell, far away from the cell surface, filaments of actin and myosin—proteins also involved in muscle cell contraction—form a dense mesh. Polymerization and contraction of this mesh trigger actin flows towards the animal pole of the egg, the hemisphere that is going to differentiate into the later embryo. Via passive frictional forces, these actin flows drag along cytoplasm. The bigger yolk granules, in contrast, are not dragged along by actin since their friction with actin is much lower. Instead, they are actively pushed, or rather squeezed, towards the opposite vegetal pole of the egg by comet-like actin structures—particular actin structures whose function had not been reported in developmental processes before. The combination of these pulling and pushing forces ensures a robust segregation of the cytoplasm and yolk granules within the developing embryo.
Bringing darkness into the light
By examining deeper parts of the cell more closely, the multi-disciplinary team has revealed that animal pole expansion at the cell surface, as previously proposed, is not essential for the yolk-cytoplasm segregation. "The actin structures at the cell surface appear very bright and are therefore quite easy to study. Maybe that's why scientists have so far simply missed to look more deeply into the much darker bulk area, which makes up most of the cell," says Shayan Shamipour, lead author of the study. Refined image processing allowed the IST Austria researchers to take a closer look at the developments in fish eggs during the moments right after fertilization. But, as Shamipour adds, another key to success was something else: "To catch the very first moments of egg development, we had to be really fast: Whenever one of our fish had started to release its eggs into the water, I would press start on my stop watch and my colleagues would see me sprint from the fish facility to the microscopy room to observe and record the process."
Curiosity-driven teamwork at its best
According to the cell biologist with a background in physics, Shamipour had been suspicious of the prevailing surface-based explanation for a while: "The embryo follows a big goal: It has to divide from one into thousands of cells in a very short amount of time. It was thus evident that the proposed surface mechanism alone could not accomplish this segregation and that the embryo would have to come up with some other mechanisms to accelerate the process." It is this curiosity-driven attitude of the young scientist paired with the interdisciplinary research culture of the Heisenberg and Hannezo groups—a mode of scientific work IST Austria particularly fosters—that enabled Shamipour to identify and analyze central cell processes that could be relevant in many other settings and organisms.
More information: Shayan Shamipour et al, Bulk Actin Dynamics Drive Phase Segregation in Zebrafish Oocytes, Cell (2019). DOI: 10.1016/j.cell.2019.04.030
Journal information: Cell
Provided by Institute of Science and Technology Austria