Performing under pressure: Modeling oxidation in high-stress materials
Each year, the effects of corroding materials sap more than $1 trillion from the global economy. As certain alloys are exposed to extreme stress and temperatures, an oxide film begins to form, causing the alloys to break down even more quickly. What precisely makes these high-temperature, high-stress conditions so conducive for corrosion, however, remains poorly understood, especially in microelectromechanical devices. In the Journal of Applied Physics, Chinese researchers have started to chip away at why these materials corrode under mechanical stress.
Xue Feng, a professor at Tsinghua University, and his research team describe how mechanical stress can affect the oxidation process. Their model draws on oxidation kinetics to explain how stress affects the oxidation species that diffuse throughout the oxide layer, and how stress modifies chemical reactions at interfaces and lead to oxidation.
"Our work is in the direction of fundamental research, but it is indeed based on engineering problems," Feng said. "We expect that it provides guidelines for more accurate predictions in engineering applications, including better designs to compensate for material and system failure by taking into account the oxidation process."
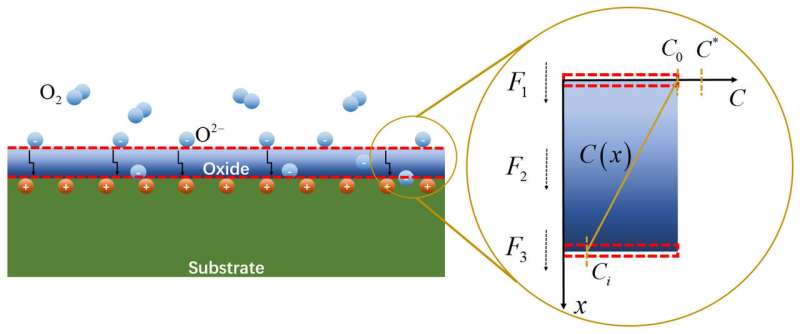
For decades, research into the chemomechanical coupling of physical stress and oxidation focused on relating stress to one of two different features of alloy corrosion. Specifically, stress tends to accelerate the oxidation occurring on the surface of the material at the interface between the device and oxygen from the surrounding air. Stress also changes the ways oxidative compounds diffuse throughout the nanoscale structure of a material.
This group's work combines stress and the oxidation process into a new model. First, a substrate, typically the corroding alloy, absorbs oxygen and forms a metal oxide layer. More oxygen can diffuse through this layer, which can react with the next layer of alloy behind the oxidation interface.
"Our work here mainly deals with the second and third stages, in which the stress, either externally applied mechanical loading or intrinsically generated stress due to the oxide formation itself, could affect the diffusion and chemical reaction process," said Mengkun Yue, another author of the paper from Tsinghua University.
The team's model predicted that when materials under heavy loads are compressed, they absorb less oxygen. Correspondingly, stresses that pull the material apart provide more space for oxygen to infiltrate the alloy.
The group tested this framework on samples of SiO2 grown on a Si substrate using multibeam interferometry, a method that other researchers had previously demonstrated, and found that their theoretical predictions matched the data.
Xufei Fang, an author on the paper at Max Planck Institute for Iron Research, said he hopes that verifying a unified model for stress-oxidation coupling can help improve microelectromechanical devices. At high temperatures or under stress, these devices can experience markedly more oxidation because of their large surface area-to-volume ratio.
"We expect a more general application of our model and we will develop our model further, in the next steps, to apply them to microscale systems," Fang said.
More information: "Effect of interface reaction and diffusion on stress-oxidation coupling at high temperature," Journal of Applied Physics (2018). DOI: 10.1063/1.5025149
Journal information: Journal of Applied Physics
Provided by American Institute of Physics