Seeing nanoscale details in mammalian cells

In 2014, W. E. Moerner, the Harry S. Mosher Professor of Chemistry at Stanford University, won the Nobel Prize in chemistry for co-developing a way of imaging shapes inside cells at very high resolution, called super-resolution microscopy. Now, he and his lab have created a new microscope that produces 3-D nanoscale images of mammalian cells in their entirety.
"A cell has a whole city of proteins, enzymes and structures working all the time," Moerner said. "We have some idea of what's in a cell – many of us are familiar with drawings of mitochondria or of the endoplasmic reticulum – but it's an average idea. When we look at individual cells, we recognize that they all aren't exactly like the pictures we have in textbooks."
The Moerner lab blends chemistry, physics, optics and engineering to create better ways of peering inside cells to see the workings of individual molecules. Collaborating with many other labs, the group focuses on biological subjects, such as measuring the structures of protein fibers related to Huntington's disease, observing the organization of individual strands of DNA in the nucleus and documenting structural changes in cells during medical treatments.
Pancakes and magic
The new microscope, which the researchers call TILT3D and which was described recently in a paper published in Nature Communications, combines two new imaging techniques with super-resolution microscopy to capture very clear 3-D images of structures and individual molecules within a cell.
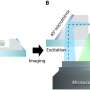
One of the two new techniques, known as tilted light sheet illumination, addresses problems with focus and functionality that occur with existing illumination techniques. In most light microscopes, the cell sample is lit from below.
"This is a problem if you want to investigate the details of a cell because it leads to visually hazy images where only some parts are in focus – like a photo taken over a long distance," said Anna-Karin Gustavsson, a postdoctoral fellow in the Moerner lab and lead author of the paper.
Standard light sheet illumination gets around this problem by shining only a slice of light in from the side to obtain a pancake-like illumination of the sample. Even with this advantage, if you try to get a light sheet to shine on the very bottom of a cell, it bounces off the corner of the chamber containing the sample, which distorts the image. By tilting the light sheet, the Moerner lab avoids hitting the corner.
In addition to clearing the visual clutter by tilting the light sheet, the new microscope includes an optical method for imaging in 3-D. To achieve this, the researchers tag molecules in the cell sample with chemicals that fluoresce when lit and use chemical additives to make them blink brightly. Then, through what Moerner calls "optical magic," the group adjusts the microscope to convert each fluorescent blink into two spots of light at different angles. With these two spots, the researchers can get the position of each molecule in three dimensions, which informs the final 3-D image.
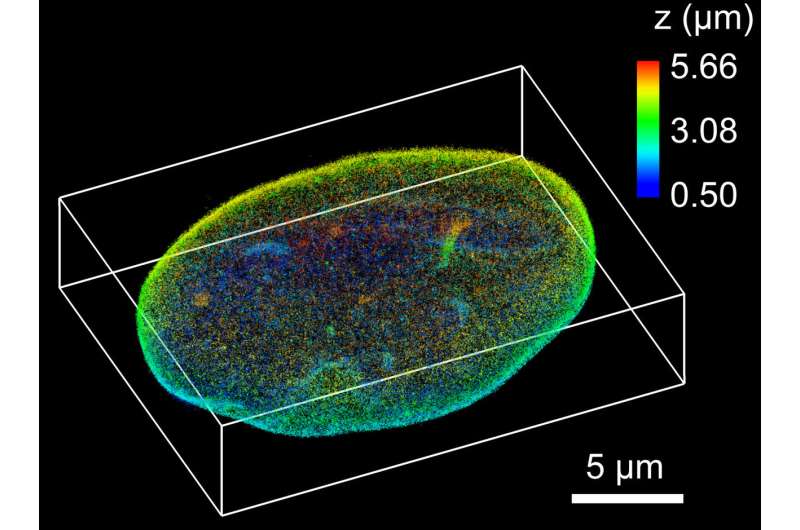
Stacking their pancaked 3-D images on top of one another, the researchers can create a top-to-bottom reconstruction of a cell. Tilted light sheet imaging also makes it possible to track the 3-D movement of molecules over time with a precision of tens of nanometers, which could capture molecules bonding, moving by motors or traveling randomly through structures of the cell.
Combining TILT3D's clear image and 3-D capabilities with existing super-resolution techniques, the microscope can create precise images at super-resolution – as small as tens of nanometers or about 4,000 times smaller than a human hair is thick. This opens up new opportunities to produce detailed 3-D images of mammalian cell structures, even of ones that were previously too dense to image clearly.
Ready to share
As part of their paper, Moerner and his lab members successfully tested their microscope on known cellular structures. They are already walking other labs through the process of duplicating this microscope. The design can be a modular addition to existing light microscopes. In the future, they hope that their 3-D tilted light sheet illumination imaging will be used for any number of projects.
"TILT3D is simpler than other microscopes that have been designed for imaging of these challenging samples, and it can be used for imaging both of static structures and of moving molecules" said Gustavsson, who is partly supported by a postdoctoral fellowship from the Karolinska Institutet in Sweden. "We designed it to be versatile, not bound to a specific question."
The researchers will continue to work on TILT3D, particularly on combining static and dynamic information from several different proteins. Alongside their many other innovations and studies in cellular imaging, they hope this technology can enable them and others to learn more about the structures and processes of cells, one molecule at a time.
More information: Anna-Karin Gustavsson et al. 3D single-molecule super-resolution microscopy with a tilted light sheet, Nature Communications (2018). DOI: 10.1038/s41467-017-02563-4
Journal information: Nature Communications
Provided by Stanford University