Light-controlled tool can be used to reveal secrets of protein function
UC San Francisco scientists have invented a technique that lets them precisely and reversibly disrupt the action of specific cellular proteins at a microscopic scale by making them split apart when illuminated with blue light. The researchers envision a vast number of applications for the technique in the study of cell biology.
"A challenge of the post-genomic era is to figure out what specific proteins do in cells, and when and where they do it, something that cannot be achieved by genetic knockouts alone," said Torsten Wittmann, Ph.D., a professor in the department of Cell and Tissue Biology in UCSF's School of Dentistry. "Here we show a new method to inactivate specific proteins acutely, locally and reversibly inside living cells, which should be able to address many of these questions."
In a new paper published Jan. 29, 2018, in Nature Cell Biology, Wittmann's team used the new tool to demonstrate for the first time that cancer cell migration depends on the ability of components of the cell's dynamic skeleton—known as microtubules—to grow in the direction of cell movement, a finding with implications for scientists' understanding of cell migration and potentially the development of new therapies to block cancer metastasis.
Biologists have developed a number of tools in recent years that allow them to manipulate cells with light. These techniques, collectively termed "optogenetics," originally involved genetically introducing specific light-sensitive proteins into brain cells to let researchers activate the cell with light. In contrast, the new photo-inactivation technique lets researchers make any of a vast number of different proteins light-sensitive in any type of cell, and allows them to switch the protein's function off and on rapidly in tightly controlled regions of the cell, which makes it an extremely powerful tool for advancing our knowledge of cell biology.
The new technology relies on a pair of proteins called LOV2 and Zdk1, which bind together in the dark, but come apart like a pair of Lego bricks when exposed to blue light. (LOV2 is derived from oat plants, where it is involved in phototropism – a plant's ability to grow towards a light source.) By inserting these two proteins – which the researchers dubbed the "photo-inactivation element" – at a key structural site within a larger protein via genetic engineering, the researchers could make that protein split in two and become inactive when exposed to blue light.
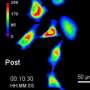
In the new paper, postdoctoral researcher Jeffrey van Haren, Ph.D., the study's first author, used the technique to investigate the role of microtubules in a cell's ability to move. In small-cell lung cancer cells, the team inserted LOV2 and Zdk1 into the protein EB1, an important regulator of microtubule growth, and showed that illuminating any part of the cell with blue light could slow microtubule growth within seconds in the illuminated region (see video). When the light was turned off, the EB1 molecules came back together, also within seconds, and allowed microtubule growth to return to normal.
Previously, the field had thought of cells' more muscular actin cytoskeleton as the key player in cell migration, Wittmann says, but the UCSF team found that they could reverse a cell's direction by preventing its microtubules from growing into the migrating cell's leading edge. In a dramatic demonstration of this finding, the researchers showed that they could completely trap a migrating cancer cell by surrounding it in a virtual cage of light.
"We believe this strategy will be useful to be able to generate many other light-inactivated proteins," Wittmann said. "A lot of cellular proteins are modular, with large folded domains tethered together by unstructured linkers. By inserting a photo-inactivation element in place of these linkers, we should be able to make any such protein sensitive to light."
Wittmann contrasts the speed and reversibility of the new photo-inactivation technique to existing genetic knock-out approaches, in which researchers eliminate specific genes from cells or whole animals in order to study the function of the proteins they produce. But it can take days or weeks for protein levels to change in those experiments, Wittmann said, which may give cells time to adapt and change their behavior in response to these genetic manipulations, researchers believe.
"Photo-inactivation lets us turn proteins off and back on in living cells in real time, and do so with much more spatial accuracy than has been possible before," Wittmann said. "I hope this becomes a key tool for anyone interested in understanding how these tiny molecular machines make every cell in our bodies tick."
More information: Local control of intracellular microtubule dynamics by EB1 photodissociation, Nature Cell Biology (2018). nature.com/articles/doi:10.1038/s41556-017-0028-5
Journal information: Nature Cell Biology
Provided by University of California, San Francisco