New reaction turns feedstock chemical into versatile, chiral building block
Researchers in the Doyle lab at Princeton have developed a direct cross-coupling reaction to produce nitrogen-containing compounds called 1,2-dihydropyridines, versatile building blocks that are highly useful in pharmaceutical research.
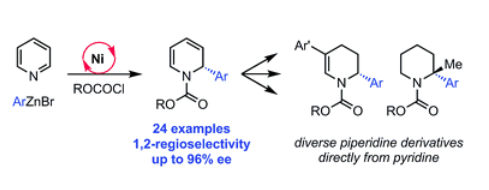
Published in Chemical Science, the reaction employs a chiral nickel catalyst and an activating agent at low temperatures to couple nucleophilic arenes, common motifs in bioactive compounds, with a feedstock chemical known as pyridine.
"A highlight of the method is being able to use pyridine as a substrate because it's inexpensive and abundant and has rarely been used in transition metal and asymmetric catalysis," said Abigail Doyle, an associate professor of chemistry at Princeton and corresponding author of the article.
Performing transition metal chemistry with pyridine has proven challenging because it can 'poison' the nickel catalyst, essentially binding to the nickel such that the reaction cannot move forward. The research team found that they could overcome this limitation by adding a slight excess of an activating agent, a compound known as iso-butylchloroformate. This addition favors the formation of an intermediate species that will not bind to the catalyst and allows the reaction to proceed.
The method is also highly enantio- and regioselective, meaning that researchers could control the precise geometry and position at which the new chemical bond is formed between the two coupling partners, attractive features that have not been offered by previous methods.
The researchers went a step further by demonstrating the utility of the product by performing nine different elaborations commonly used by medicinal chemists in drug development. "That was my favorite part to do," said Patrick Lutz, a graduate student in the Doyle lab and lead author of the paper. "Optimization is necessary, and exciting when you find ways to improve the reaction, but it was really fun thinking about all the different types of reactions that I could do."
More information: J. Patrick Lutz et al. Nickel-catalyzed enantioselective arylation of pyridine, Chem. Sci. (2016). DOI: 10.1039/C6SC00702C
Journal information: Chemical Science
Provided by Princeton University