A method to identify human induced pluripotent stem cells in the lab using a tumor rejection antigen
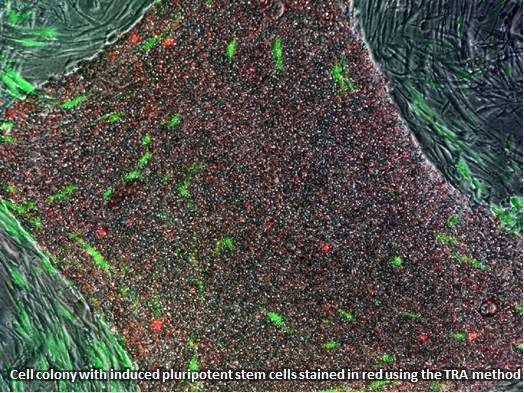
This week, the United States Patent and Trademark Office issued a major patent to two scientists from the UCLA Eli & Edythe Broad Center of Regenerative Medicine & Stem Cell Research, William Lowry, Ph.D., and Kathrin Plath, Ph.D. The patent covers a method used to identify human induced pluripotent stem cells in the lab using a tumor rejection antigen, or TRA, which identifies stem cells that have reached a pluripotent state.
"Using the TRA method is an important validation step to ensure that the target cells have potentially been reprogrammed to a pluripotent state," said Plath, professor of biological chemistry at UCLA. "This accurate identification of reprogrammed cells is critical as we conduct research to better understand the viability of pluripotent stem cells for therapeutic treatments for human disease."
The vast majority of human pluripotent stem cells used in scientific research today are created by either using a donated human embryo (called human embryonic stem cells or hESC) or using a patient's own cells (called induced pluripotent stem cells or iPSC). Both hESC and iPSC are considered 'pluripotent' because they self-renew or replicate and have the capability to turn into any other cell in the body. Human embryonic stem cells are obtained from stored frozen embryos donated by individuals or couples who have completed in vitro fertilization. Induced pluripotent stem cells are adult skin or blood cells that are reprogrammed back into an embryonic-like state, giving them the same regenerative properties without the use of a donated embryo. In late 2007, a team of scientists from the UCLA Broad Stem Cell Research Center, including Plath and Lowry, were the first in California to create human iPSC.
One challenge with hESC is that they are not genetically matched to the patient. Therefore, as with any other type of medical transplant, patients receiving hESC therapies must also take anti-rejection medication. Made from a patient's own cells, iPSC are perfectly matched to that patient and theoretically avoid the risk of rejection. Nevertheless, some risks have been identified in the reprogramming process. Since iPSC do not start in a pluripotent state like hESC, scientists must validate that the donor cells, such as skin or blood, have been reprogrammed into iPSC to ensure the right kinds of cells are used in their research. Furthermore, as stem cell science moves from the lab to viable clinical treatments, validating the cells has become a critical step in ensuring stem cell treatments are ready for human clinical trials.
"Both hESC and iPSC are currently being used in scientific studies that do not include humans," said Lowry, associate professor of molecular, cell and developmental biology in the life sciences at UCLA. "However, when it comes to human trials that involve pluripotent stem cells, almost all of those trials use hESC. As we work to understand if iPSC are also safe for human trials, it's vitally important that we're working with the right cells. That's where the TRA method comes in."
Prior to the use of the TRA method, the process of picking pluripotent stem cells out of the other cellular matter contained in the petri dish was a lot like trying to find a needle in a haystack. Scientists reprogrammed adult cells with the intent to achieve pluripotency, but in order to validate the pluripotent state of the cells, they had to be stained in a way that caused them to die. As a result, scientists had to use a complex duplication system to keep a replicate of the stem cell collection they were testing so they would still have living cells once pluripotency was established.
Pluripotent stem cells can be recognized by the TRA antibody. When added to reprogrammed human cells in a petri dish, the antibody recognizes and binds to a molecule that only exists on human pluripotent stem cells. Attaching a color to the antibody stains the pluripotent cells without harming them. This method allows for easy viewing and results in accurate identification and selection of potentially pluripotent cells. The TRA staining method is now the standard first step in most research studies involving iPSC.
Provided by University of California, Los Angeles