Chemical engineers borrow technique to store solar energy
(Phys.org) —Chemical engineers at Stanford have designed a catalyst that could help produce vast quantities of pure hydrogen through electrolysis – the process of passing electricity through water to break hydrogen loose from oxygen in H2O.
Today, pure hydrogen, or H2, is a major commodity chemical that is generally derived from natural gas. Tens of millions of tons of hydrogen are produced each year; industrial hydrogen is important in petroleum refining and fertilizer production.
Chemical engineering Professor Thomas Jaramillo and research associate Jakob Kibsgaard want to use electrolysis to do things such as producing H2 from water and using the process to store solar energy. But to industrialize water-splitting they must find a more cost-effective process.
Electrolysis in classroom experiments is simple: lower two metal electrodes into water; when electricity is passed through these electrodes they act as catalysts to break water molecules into bubbles of hydrogen and oxygen gas.
Platinum is the best catalyst for producing hydrogen through water electrolysis. But to make electrolysis an industrial process a cheaper electrode must be found. "We're trying to make H2 in the most efficient way possible without using precious metals," Jaramillo said.
In the German scientific journal Angewandte Chemie, Jaramillo and Kibsgaard describe a cheap, durable and efficient catalyst that could take the place of platinum.
Their ambitions go beyond using electrolysis merely to replace the current market demand for hydrogen.
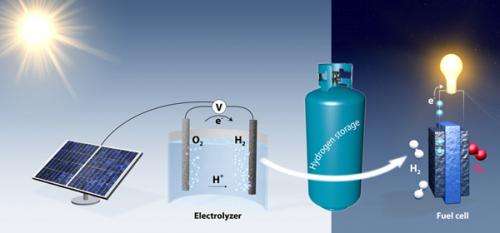
Right now there is no cost-effective, large-scale way to store solar energy. The Stanford researchers believe that electrolysis could turn tanks of water into batteries for storing solar energy. During the day, electricity from solar cells could be used to break apart water into hydrogen and oxygen. Recombining these gases would generate electricity for use at night.
Electrolysis uses electricity to crack the chemical bonds that hold H2O together.
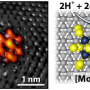
Cracking the chemical bonds of water produces a hydrogen ion – a proton with no electron to balance it out. A good H2 catalyst gives the proton a place to stick until it can pick up an electron to form a hydrogen atom on the catalyst surface and then pair up with a neighboring hydrogen atom to bubble off as H2.
The trick is finding a catalyst with the right stickiness.
"If the binding is too weak, the ions don't stick," Jaramillo said. "If it's too strong, they never get released."
Platinum is perfect but pricey. Last year the Stanford engineers discovered that a version of molybdenum sulfide, a catalyst widely used in petrochemical processing, had some of the right properties to serve as a cheap but efficient alternative to platinum.
Jaramillo explained that petrochemical processing has similarities to electrolysis. That's because petroleum feed stocks, such as tar sands, contain a significant fraction of heavy molecules. Petroleum refineries use catalytic reactions that involve hydrogen to crack these heavy molecules into lighter molecules like gasoline.
Similarly, electrolysis involves cracking water molecules, or breaking apart their chemical bonds. As the Stanford engineers sought to improve on their own discovery they found an even better way to produce hydrogen from water by taking yet another page from the petrochemical playbook.
Petroleum processing often involves scrubbing sulfur out of fuels to reduce acid rain. During this scrubbing process, some of the sulfur atoms get incorporated into petroleum processing catalysts, increasing the activity of these catalysts.
This gave the Stanford engineers an idea: If they laced an already good catalyst with sulfur atoms, would it become an even better electrode for producing pure hydrogen?
They chose to add sulfur atoms to a catalyst called molybdenum phosphide, which is known to speed up hydrogen production though electrolysis.
Adding the sulfur atoms created a new catalyst – molybdenum phosphosulfide– that was more effective at producing hydrogen than its predecessor.
The new sulfur-laced catalyst was more durable, which is vital in an industrial process where the electrode must function day in, day out, without degrading, just like the noble metal platinum.
The molybdenum phosphosulfide catalysts developed by Kibsgaard and Jaramillo are a major advance. As electrodes they are remarkably stable with an efficiency approaching that of platinum.
Now, members of Jaramillo's group are working to improve this new catalyst. For instance they are engineering the material at nano-scale dimensions to catalyze the reaction more effectively. Other research initiatives include incorporating this catalyst into bench-top prototypes of future energy storage systems. The idea would be to use water electrolysis to store solar energy by day in the form of H2 and then, at night, to recombine hydrogen and oxygen into water, generating electricity in the process.
Jaramillo noted that the findings in this and the prior scientific paper pursue environmentally friendly energy strategies, but they are based on ideas borrowed from petrochemical plants.
"It's exciting to make these connections between really different areas of technology," he said, "and aim to operate at the meta-level of science."
More information: "Molybdenum Phosphosulfide: An Active, Acid-Stable, Earth-Abundant Catalyst for the Hydrogen Evolution Reaction." Angew. Chem. Int. Ed.. doi: 10.1002/anie.201408222
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Stanford University