Researchers turn harmful greenhouse gas into a tool for making pharmaceuticals
A team of chemists at USC has developed a way to transform a hitherto useless ozone-destroying greenhouse gas that is the byproduct of Teflon manufacture and transform it into reagents for producing pharmaceuticals.
The team will publish their discovery in a paper entitled "Taming of Fluoroform (CF3H): Direct Nucleophilic Trifluoromethylation of Si, B, S and C Centers," in the Dec. 7 issue of Science.
The method is also being patented.
Because of the popularity of Teflon, which is used on everything from cooking pans to armor-piercing bullets, there's no shortage of its waste byproduct, fluoroform. Major chemical companies such as DuPont, Arkema and others have huge tanks of it, unable to simply release it because of the potential damage to the environment. Fluoroform has an estimated global warming potential 11,700 times higher than carbon dioxide.
But one man's trash is another man's treasure, and G.K. Surya Prakash—who has spent decades working with fluorine reagents—saw the tanks of fluoroform as an untapped opportunity.
Prakash, a professor of chemistry at the USC Dornsife College of Letters, Arts and Sciences and director of the USC Loker Hydrocarbon Research Institute, describes fluorine as "the kingpin of drug discovery." About 20 to 25 percent of drugs on the market today contain at least one fluorine atom.
Fluorine can be found in all different kinds of drugs, everything from 5-Fluorouracil (a widely used cancer treatment discovered by Charles Heidelberger at USC in the '70s) to Prozac to Celebrex.
"It's a small atom with a big ego," he said, referring to the fact that while fluorine is about the same size as a tiny hydrogen atom—so similar that living cells cannot tell the two elements apart—it is also extremely electronegative (that is, it has a strong attraction for electrons) making carbon-fluorine chemical bond quite strong, which improves the bioavailability of drugs made with fluorine.
Prakash led a team that included long-time colleague George A. Olah, distinguished professor of chemistry at USC Dornsife, and USC Research Associates, Parag V. Jog and Patrice T. D. Batamack.
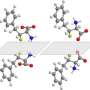
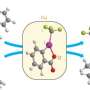
The discovery was the product of many years of trial-and-error tests, hard work that the postdocs performed under Prakash's direction. Eventually, the team pinned down the precise conditions needed to coax the harmful fluoroform (CF3H) into useful reagents, including the silicon-based Ruppert-Prakash Reagent for efficient CF3 transfer. Fluoroform with elemental sulfur was also converted to trifluoromethanesulfonic acid, a widely used superacid one-hundred times stronger than sulfuric acid.
"In real estate, everything is 'location, location, location.' In chemistry, it is 'conditions, conditions, conditions,'" Prakash said.
More information: "Taming of Fluoroform: Direct Nucleophilic Trifluoromethylation of Si, B, S, and C Centers," "by G.K.S. Prakash, Science, 2012.
Journal information: Science
Provided by University of Southern California