Introduction of fluorine atoms into organic molecules could have applications for synthesis of pharmaceuticals
Despite its small size, the fluorine atom has had a vast impact on the pharmaceutical industry. More often than not, introducing fluorine to a drug molecule improves the drug's biological activity, earning it a reputation as a 'magic element'.
Amino acids—comprising an amino group and a carboxylic acid group—are also important to the medicinal chemist. Amino acids are not only the building blocks of proteins, they are commonly found in pharmaceutical drugs. Now, Mikiko Sodeoka and colleagues at the RIKEN Advanced Science Institute, Wako, have developed a synthesis technique that can selectively and efficiently combine fluorine and amino acids into the same organic molecule.
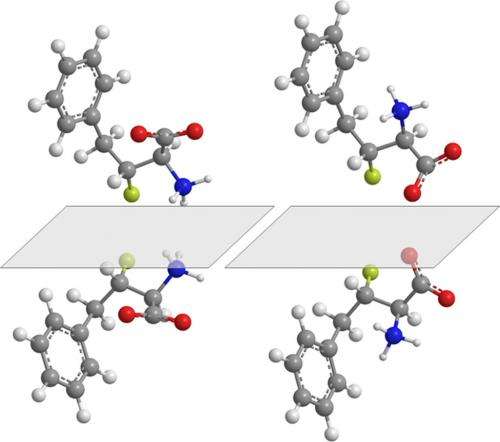
The starting materials are alpha-keto esters that contain a carbonyl group and an ester group. The first reaction of the team's technique is the substitution of a hydrogen atom, on the carbon atom adjacent to the carbonyl group, for a fluorine atom. As there are two hydrogen atoms that could be replaced, two mirror images, or enantiomers could result. Sodeoka and colleagues use a palladium catalyst that preferentially forms one of these enantiomers. This renders the reaction enantioselective; that is, one enantiomer is selectively formed over the other. The carbon atom to which the fluorine attaches becomes a stereogenic center as it has four different substituents. The interchange of any two substituents gives a pair of enantiomers. In the wider picture, these enantiomers are stereoisomers—molecules that differ only by their 3D orientation of the atoms.
Next, the carbonyl group of the fluorinated alpha-keto ester transforms to a hydroxyl group. Again, two possible stereoisomers of the molecule could form. By exploiting the existing stereogenic center and using different reagents, the researchers could synthesize one stereoisomer in preference to the other. Hence, the technique not only introduces fluorine, but two stereogenic centers to the molecule. The formation of two stereogenic centers creates the possibility of four different stereoisomers (Fig. 1). By tuning the reagents, the team isolated all four stereoisomers in separate reaction sequences.
Overcoming the chemical instability of the intermediate, however, is challenging. "The fluorinated alpha-keto esters easily convert to their hydrated form, so care is required to exclude water from the reaction mixture," Sodeoka explains. "However, the hydroxy and amino acid derivatives are stable and easy to handle."
In the future, Sodeoka and colleagues hope to widen the scope of the fluorination reaction to other starting materials. This would create the possibility of making numerous biologically active compounds.
More information: Suzuki, S., Kitamura, Y., Lectard, S., Hamashima, Y. & Sodeoka, M. Catalytic asymmetric mono-fluorination of α-keto esters: Synthesis of optically active β-fluoro-α-hydroxy and β-fluoro-α-amino acid derivatives. Angewandte Chemie International Edition 51, 4581–4585 (2012). dx.doi.org/10.1002/anie.201201303
Journal information: Angewandte Chemie International Edition
Provided by RIKEN















