Scientists bring the heat to refine renewable biofuel production

(Phys.org)—Perhaps inspired by Arizona's blazing summers, Arizona State University scientists have developed a new method that relies on heat to improve the yield and lower the costs of high-energy biofuels production, making renewable energy production more of an everyday reality.
ASU has been at the forefront of algal research for renewable energy production. Since 2007, with support from federal, state and industry funding, ASU has spearheaded several projects that utilize photosynthetic microbes, called cyanobacteria, as a potential new source of renewable, carbon-neutral fuels. Efforts have focused on developing cyanobacteria as a feedstock for biodiesel production, as well as benchtop and large-scale photobioreactors to optimize growth and production.
ASU Biodesign Institute researcher Roy Curtiss, a microbiologist who uses genetic engineering of bacteria to develop new vaccines, has adapted a similar approach to make better biofuel-producing cyanobacteria.
"We keep trying to reach ever deeper into our genetic bag of tricks and optimize bacterial metabolic engineering to develop an economically viable, truly green route for biofuel production," said Roy Curtiss, director of the Biodesign Institute's Centers for Infectious Diseases and Vaccinology and Microbial Genetic Engineering as well as professor in the School of Life Sciences.
Cyanobacteria are like plants, dependent upon renewable ingredients including sunlight, carbon dioxide and water that, through genetic engineering, can be altered to favor biodiesel production. Cyanobacteria offer attractive advantages over the use of plants like corn or switchgrass, producing many times the energy yield with energy input from the sun and without the necessity of taking arable cropland out of production.
Colleague Xinyao Liu and Curtiss have spent the last few years modifying these microbes. Their goal is to bypass costly processing steps (such as cell disruption, filtration) for optimal cyanobacterial biofuel production.
"We wanted to develop strains of cyanobacteria that basically can process themselves," said Curtiss. "A couple of years ago, we developed a Green Recovery process that is triggered by removing carbon dioxide to control the synthesis of enzymes, called lipases, that degrade the cell membranes and release the microbes' precious cargo of free fatty acids that can be converted to biofuels,"
However, when growth of cyanobacteria is scaled up to meet industrial needs, they become dense, and the self-shading that occurs in concentrated cultures, does not let in enough light to produce enough of the lipases to efficiently drive the process. Thus the original Green Recovery was light dependent and maximally efficient at sub-optimal culture densities.
Curtiss' team looked again at nature to improve their Green Recovery method. The process uses enzymes found in nature called thermostable lipases synthesized by thermophilic organisms that grow at high temperatures such as in hot springs. These thermostable lipases break down fats and membrane lipids into the fatty acid biodiesel precursors, but only at high temperatures. The team's new process, called thermorecovery, uses a heat-triggered, self-destruct system. By taking a culture and shifting to a high temperature, the lipases are called into action. This process occurs with concentrated cultures in the dark under conditions that would be very favorable for an industrial process.
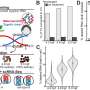
They tested a total of 7 different lipases from microbes that thrive in hot springs under very high temperatures, a scorching 60-70 C (158F). The research team swapped each lipase gene into a cyanobacteria strain that grows normally at 30 C (86 F) and tested the new strains.
They found the Fnl lipase from Feridobacterium nodosum, an extremophile found in the hot springs of New Zealand, released the most fatty acids. The highest yield occurred when the carbon dioxide was removed from the cells for one day (to turn on the genes making the lipases), then treated at 46C (114F) for two days (for maximum lipase activity).
The yield was 15 percent higher than the Green Recovery method, and because there were less reagents used, time (one day for thermorecovery vs. one week for Green Recovery) and space for the recovery. Thermorecovery resulted in an estimated 80% cost savings.
Furthermore, in a continuous semi-batch production experiment, the team showed that daily harvested cultures released could release a high level of fatty acid and the productivity could last for at least 20 days. Finally, the water critical to growing the cultures could be recycled to maintain the growth of the original culture.
"Our latest results are encouraging and we are confident of making further improvements to achieve enhanced productivity in strains currently under construction and development," said Curtiss. "In addition, optimizing growth conditions associated with scale-up will also improve productivity."
More information: Journal of Biotechnology Volume 161, Issue 4, 15 November 2012, Pages 445–449. doi: 10.1016/j.jbiotec.2012.08.013
Journal information: Journal of Biotechnology
Provided by Arizona State University