Fuel cells operating directly on ethanol

(Phys.org) -- Researchers at the Center for Energy Research at UC San Diego recently demonstrated the best performance for solid oxide fuel cells (SOFCs) operating directly on ethanol without external reformation. The work was performed by Dr. Nguyen Minh of the Center for Energy Research, postdoctoral scholar Dr. Eric Armstrong (now with Intel) and UC San Diego undergraduate student intern Jae-Woo Park.
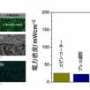
A peak power density of more than 400 mW/cm2 was achieved at 800°C with air and a fuel containing 7.3 volume percent ethanol. This power density is about four times higher than any other SOFC reported in the literature operating directly on ethanol at 20 volume percent or lower at the same temperature
The SOFC is an all-solid-state fuel cell consisting of an ionic conducting oxide electrolyte sandwiched between two electrodes, the cathode or oxygen electrode where oxygen (from air) is reduced and the anode or fuel electrode where hydrogen (from the fuel) is oxidized.
This type of fuel cell operates in the temperature range of 600°-1000°C. At present, the most common SOFC materials are yttria stabilized zirconia (YSZ) (an oxygen ion conductor) for the electrolyte, strontium-doped lanthanum manganite perovskite oxide (LSM) for the cathode and nickel/YSZ composite for the anode. The attractive feature of the SOFC is its clean and efficient generation of electricity from a variety of fuels. Suitable fuels for the SOFC include hydrogen, natural gas, biogas, propane, gasoline, diesel, coal gas, and other practical fuels. The SOFC has been considered and developed for a broad spectrum of power generation applications, ranging from watt-size portable devices to multi-megawatt baseload power plants. In addition, the operation of the SOFC is reversible, i.e. the fuel cell can operate in reverse or electrolysis mode when integrated with an energy source. Thus, the SOFC can be used as an electrolysis cell to produce hydrogen from water or syngas (mixtures of hydrogen and carbon monoxide) from mixtures of water and carbon dioxide. A SOFC can operate efficiently in both operating modes is referred to as a reversible SOFC.
The SOFC has been shown to be capable of directly utilizing hydrocarbons and other fuels such as alcohols without external reformation. SOFC power systems based on direct utilization do not require an external reformer, thus simplifying the system, resulting in higher system efficiencies and reduced costs. Nickel in the anode, although an excellent catalyst for hydrogen oxidation, tends to promote coking. Therefore, for direct utilization of carbon-containing fuels, copper/ceria (Cu/CeO2) composites have been proposed and investigated. The copper/ceria composite is resistant to coking; however, its catalytic activity for hydrogen oxidation is much lower than that of Ni/YSZ. Thus, direct utilization of non-hydrogen fuels on copper/ceria often results in poor electrochemical performance.
The approach at CER to address the electrochemical performance and coking issues to demonstrate the feasibility of direct utilization SOFCs (referred to as direct SOFCs) is to engineer the anode structure into a dual (bifunctional bilayer) anode. The engineered anode structure is composed of a Ni/YSZ support outer layer impregnated with Cu/ceria nanoparticles (see scanning electron microscopy or SEM photographs) to promote reformation and minimize coking and a thinner Ni-YSZ electroactive interlayer (next to the electrolyte) to maintain high electrochemical performance. The fabrication of SOFC cells incorporating this anode structure was straightforward. Cells with dual anode layers were first fabricated using the conventional materials and techniques (tape casting and sintering). The outer anode layer of fabricated cells was then impregnated with an aqueous solution of copper and cerium nitrates of appropriate weight ratios, followed by high temperature (850°C) annealing to form oxide nanoparticles. (Nickel and copper were formed as oxides in this case and the oxides were reduced to metal when fuel was introduced to the anode.)
Provided by University of California - San Diego