November 22, 2011 weblog
Battery electrode's 40,000 charge cycles look promising for grid storage

(PhysOrg.com) -- While researchers continue to improve solar and wind energy technologies, having a battery that can store the energy until it’s needed by the grid is another critical component for using renewable energy on a large scale.
One of the biggest problems researchers face is designing an inexpensive battery that can be recharged many times. Today’s lead-acid batteries typically last only a few hundred charge cycles, while lithium-ion batteries can last up to 1,000 charge cycles.
Now in a new study, researchers led by Yi Cui, a materials science and engineering professor at Stanford University, have designed and demonstrated a battery cathode that lasts for 40,000 charge cycles while maintaining 83% of its charge-holding capacity. The results are published today in Nature Communications.
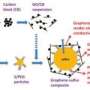
The cathode consists of commonly available materials: crystalline nanoparticles made of Prussian Blue (an iron and cyanide compound) in which half of the iron is replaced with copper. The nanoparticles are then coated on a carbon substrate, which is immersed in an electrolyte solution of potassium nitrate.
The researchers plan to design similar anodes to go along with the cathodes, which could then be used in a battery that Cui’s team is working on. That battery’s chemistry is similar to that of lithium-ion batteries but uses materials that are cheaper and more abundant, such as sodium and potassium ions and a water-based electrolyte, rather than organic solvent-based electrolytes.
In addition to its high number of charge cycles, the cathode also has high efficiency of 99%. Although the cathode’s charge capacity is not as high as that of other batteries, its overall performance in a future battery could still make it advantageous in terms of overall cost.
More information: Colin D. Wessells, et al. "Copper hexacyanoferrate battery electrodes with long cycle life and high power." Nature Communications 2, Article number: 550, DOI:10.1038/ncomms1563
Below is a press release issued by Stanford University:
Nanoparticle electrode for batteries could make grid-scale power storage feasible
The sun doesn't always shine and the breeze doesn't always blow and therein lie perhaps the biggest hurdles to making wind and solar power usable on a grand scale. If only there were an efficient, durable, high-power, rechargeable battery we could use to store large quantities of excess power generated on windy or sunny days until we needed it. And as long as we're fantasizing, let's imagine the battery is cheap to build, too.
Now Stanford researchers have developed part of that dream battery, a new electrode that employs crystalline nanoparticles of a copper compound.
In laboratory tests, the electrode survived 40,000 cycles of charging and discharging, after which it could still be charged to more than 80 percent of its original charge capacity. For comparison, the average lithium ion battery can handle about 400 charge/discharge cycles before it deteriorates too much to be of practical use.
"At a rate of several cycles per day, this electrode would have a good 30 years of useful life on the electrical grid," said Colin Wessells, a graduate student in materials science and engineering who is the lead author of a paper describing the research, published this week in Nature Communications.
"That is a breakthrough performance – a battery that will keep running for tens of thousands of cycles and never fail," said Yi Cui, an associate professor of materials science and engineering, who is Wessell's adviser and a coauthor of the paper.
The electrode's durability derives from the atomic structure of the crystalline copper hexacyanoferrate used to make it. The crystals have an open framework that allows ions – electrically charged particles whose movements en masse either charge or discharge a battery – to easily go in and out without damaging the electrode. Most batteries fail because of accumulated damage to an electrode's crystal structure.
Because the ions can move so freely, the electrode's cycle of charging and discharging is extremely fast, which is important because the power you get out of a battery is proportional to how fast you can discharge the electrode.
To maximize the benefit of the open structure, the researchers needed to use the right size ions. Too big and the ions would tend to get stuck and could damage the crystal structure when they moved in and out of the electrode. Too small and they might end up sticking to one side of the open spaces between atoms, instead of easily passing through. The right-sized ion turned out to be hydrated potassium, a much better fit compared with other hydrated ions such as sodium and lithium.
"It fits perfectly – really, really nicely," said Cui. "Potassium will just zoom in and zoom out, so you can have an extremely high-power battery."
The speed of the electrode is further enhanced because the particles of electrode material that Wessell synthesized are tiny even by nanoparticle standards – a mere 100 atoms across.
Those modest dimensions mean the ions don't have to travel very far into the electrode to react with active sites in a particle to charge the electrode to its maximum capacity, or to get back out during discharge.
A lot of recent research on batteries, including other work done by Cui's research group, has focused on lithium ion batteries, which have a high energy density – meaning they hold a lot of charge for their size. That makes them great for portable electronics such as laptop computers.
But energy density really doesn't matter as much when you're talking about storage on the power grid. You could have a battery as big as a house since it doesn't need to be portable. Cost is a greater concern.
Some of the components in lithium ion batteries are expensive and no one knows for certain that making the batteries on a scale for use in the power grid will ever be economical.
"We decided we needed to develop a 'new chemistry' if we were going to make low-cost batteries and battery electrodes for the power grid," Wessells said.
The researchers chose to use a water-based electrolyte, which Wessells described as "basically free compared to the cost of an organic electrolyte" such as is used in lithium ion batteries. They made the battery electric materials from readily available precursors such as iron, copper, carbon and nitrogen – all of which are extremely inexpensive compared with lithium.
The sole significant limitation to the new electrode is that its chemical properties cause it to be usable only as a high voltage electrode. But every battery needs two electrodes – a high voltage cathode and a low voltage anode – in order to create the voltage difference that produces electricity. The researchers need to find another material to use for the anode before they can build an actual battery.
But Cui said they have already been investigating various materials for an anode and have some promising candidates.
Even though they haven't constructed a full battery yet, the performance of the new electrode is so superior to any other existing battery electrode that Robert Huggins, an emeritus professor of materials science and engineering who worked on the project, said the electrode "leads to a promising electrochemical solution to the extremely important problem of the large number of sharp drop-offs in the output of wind and solar systems" that result from events as simple and commonplace as a cloud passing over a solar farm.
Cui and Wessells noted that other electrode materials have been developed that show tremendous promise in laboratory testing but would be difficult to produce commercially. That should not be a problem with their electrode.
Wessells has been able to readily synthesize the electrode material in gram quantities in the lab. He said the process should easily be scaled up to commercial levels of production.
"We put chemicals in a flask and you get this electrode material. You can do that on any scale," he said.
"There are no technical challenges to producing this on a big-enough scale to actually build a real battery."
Huggins is a coauthor of the Nature Communications paper.
© 2011 PhysOrg.com